Achievements
ADSORPTION OF AMMONIA-NITROGEN IN LANDFILL LEACHATE ON BEACH WATER-BEARING MEDIA
Li Hai-ming 1,2, Zheng
Xi-lai1, Liu Zhan-guang 2, Liu Xian-bin2
(1) Institute of Environmental
Science and Engineering, Ocean University of China, Qingdao 266071
,China;
(2) Institute of Marine Science &
Engineering , Tianjin University of Science &Technology, Tianjin 300457
,China)
Abstract:On the basis of
the field investigation of ammonia-nitrogen contaminated by landfills in the Tianjin shallow
groundwater, two representative water-bearing media are sampled in the Tianjin coastal
plain, and their major physico-chemical properties are analyzed. In addition,
dynamic curves and isotherms of ammonia-nitrogen adsorption on water-bearing
media have been measured. At last, retard coefficients(R) of ammonia-nitrogen
are calculated by means of columns experimental data. The studies show the
equilibrium period of the adsorption is 20-24 hours, and all the adsorption
isotherms are Langmuir curves. The saturated capabilityunit weight
decontaminated to ammonia-nitrogen of the silty sand and the silty soil are
0.771mg/g and 0.755mg/g respectively. The retard coefficient of the silty sand
and the silty soil are 5.00 and 4.85 respectively, which means ammonia-
nitrogen in landfill leachate can be adsorbed strongly by the water-bearing
media when leachate penetrates into the subsurface of the coastal
plain. As a result, the ammonia-nitrogen can difficultly migrate into the
water-bearing in the Tianjin coastal plain.
Keywords: Littoral area; landfill leachate; water-bearing media; ammonia-nitrogen;
adsorption
1 Introduction
Sanitary landfills have been, and continue to be, an economical method for solid waste disposal. In China, until very recently, few municipal landfills were lined. The older landfills typically have no liner and a great reliance is placed upon the natural attenuation of contaminants in the underlying unsaturated soils and the groundwater aquifer by biological and physico-chemical processes. Leakage of inorganic and organic pollutants from unlined landfills over time can influence the groundwater quality and may pose a threat to drinking water resources. The transport of landfill leachate in soils is subject to various physical, chemical and biological processes that affect the eventual concentration of pollutants in soils and groundwater. Understanding the movement of leachate in soil is essential to predicting the potential for groundwater pollution from landfills(Islam,2002).
High concentrated ammonia-nitrogen
is the principal water quality character of landfill leachate (Zhang,2000;Liu,2001;Jiang,2001;Guo,2002;Zhao,2002;Wen et al.,2004). (Wang,
1992;Xia et al.,2001; Wen et al.,2004) evaluated by an analysis of
characteristics of landfill leachate and an assessment of the potential effects
of nitrogen contamination on groundwater, which results showed that there were
large amounts of ammonia-nitrogen in landfill leachate and the contamination of
ammonia-nitrogen was the major form of pollution in groundwater of landfill. Kimmel
and braids (1980) suggested that NH4, when migrating into
aerobic zones, would be oxidized resulting in elevated NO3- concentrations.
However, no clear filed evidence was presented. Dissociation of NH4 to
free ammonia is not very likely in aquifers(Stumm and Morgan,1996). The
disappearance of NH4 coincided with
increased Mn concentrations in the plume, but the processes involved are not
understood(Ludvigsen et al.,1998).On the basis of water and pollutants
movement, transformation simulation test under the condition of different soil
columns,a dynamic model
for the soil-groundwater system was described.The model includes 4
pollutents(organic N, NH4, NO2, NO3)transport
and transformation in the unsaturated and saturated zone.The integrated
simulation model was used to simulate wastewater and pollutants movement under
the condition of landfill in Laogang town,Shanghai City (Wang et
al.,1998). Wen et al. (2004) studied the effects of waste leachate on the ammonia-nitrogen
adsorption ability in the soil nearby municipal waste landfill. The results
showed that when large amounts of organic matter and metal ions coexisted with
ammonia-nitrogen in the soil, it could decrease the adsorption ability of soil
to ammonia-nitrogen. In addition, the high concentration of ammonia-nitrogen in
the landfill the soil could inhibit the nitrifying microorganisms in the soil
and decreased the transformation speed from ammonia-nitrogen to nitrate,
resulting in that the nitration of soil has been restrained.
The ammonia-nitrogen in leachate is
known to penetrate into the water-bearing media of subsurface associated
movement of leachate. In this process, ammonia-nitrogen is
adsorbed by solid particles. Thus, it migrates in the water-bearing media retardingly
comparing with leachate moves, when the more adsorption quantity
is, more retarded action is. The adsorption isotherms of ammonia-nitrogen have
been set up through tests, but very few people studied the adsorption of high
concentrated ammonia-nitrogen in leachate on the water-bearing
media, particularly strand plain of Tianjin.In this paper, adsorption kinetics
equation and isotherms of ammonia-nitrogen are obtained by means of
the tests.Retard coefficients(R) of ammonia-nitrogen in water-bearing
media through column tests have combined convection-dispersion press
of contaminants in soil-water systems with adsorption process, which is very
important to predicting the potential for groundwater pollution from
landfills.
2 The Background
of the Research Areas
Tianjin is
situated in northeast of North China plain, southern of Yanshan ’s mountain
chain and west of Bohai Bay. The study area is a topography high in
north and low in south, whose topographical features are mountain, hills,
plain, littoral tidal flat from north to south. The study area is typically
characteristic of a climate with warm and half wettish continental monsoon with
an annual average temperature of 11 degrees centigrade , average evaporation
of 1683 mm and average rainfall of 590.1 mm, over 80
percent of which comes between June and September.
Shallow
groundwater is mainly Quaternary pore water, which the phreatic water changes
gradually the weak-confined water downward from surface. Generally, from north
to south, the aquiferous lithology changes from rough to fine, while its layer
varies gradually from mono-layer to multi-layer. Groundwater generally follows
the same course from north to south. Sand layers, well penetrated, distribute
in the foot of the mountain with water replacing intensively. In this district,
phreatic water is mainly extracted.
From north to
south, the hydrochemistry distribution characteristic of shallow groundwater is
horizontal zoning, which hydrochemistry type changes from HCO3-Ca to
HCO3-Na, HCO3.Cl-Na, and total dissolved solids (TDS)
changes from 1g/L to 10 g/L, but exceeds to 30 g/L in coast
local areas.
3 Adsorption
Equilibrium of Ammonia-Nitrogen
3.1 Characteristic
of landfill leachate
Landfill leachate
were collected from a landfill in Tianjin, which pre-precipitated 24 hours
before experimenting. Physical chemistry properties of the landfill leachate
see table 1 which shows that the principal water quality characteristic of
landfill leachate is high concentrated ammonia-nitrogen, Cl-,TDS,
total hardness (TH), CODcr、BOD5
Tab.1 Physical chemistry
properties of the landfill leachate
|
index |
content(mg/L) |
index |
content(mg/L) |
|
pH |
8.64 |
CODcr |
807.2 |
|
DO |
0.06 mg/L |
BOD5 |
449.5 |
|
TDS |
4145 mg/L |
TP |
8.5 |
|
Cl- |
868.5 |
TN |
175.6 |
|
TH(CaCO3) |
1722 mg/L |
NH3-N |
139.1 |
3.2
Physico-chemical properties of the water-bearing media
All the water-bearing media samples were collected from the strand plain of Tianjin. Considering the structure and compositions of the strand plain, two samples were selected respectively in depths of 10 and 20 m. The mechanical composition of the water-bearing media was analyzed with eyedropper method. At the same time, organic content, bulk density, porosity of the soils was analyzed. The mechanical composition and main characteristics for the water-bearing media samples are presented in Table 2.
Table 2 Mechanical composition and major
characteristics of selected water-bearing media
|
Sample code |
Soil mechanic composition
(%) |
Organic content (%) |
Bulk density(g/cm3) |
specific gravity(g/cm3) |
Porosity |
Soil types |
|||
|
(<0.005mm) |
(0.005~ 0.075mm) |
(0.075~0.25mm) |
(0.25~ 0.5mm) |
||||||
|
FS |
5.57 |
34.00 |
58.01 |
2.43 |
0.27 |
1.68 |
2.04 |
0.37 |
Silty sand |
|
FT |
6.74 |
74.15 |
19.12 |
0 |
0.58 |
1.64 |
2.03 |
0.39 |
Silty soil |
3.3 Determination
of adsorptive dynamic curves of ammonia-nitrogen
In order to
determine the adsorption equilibrium time of ammonia-nitrogen, an adsorption
test was conducted with silt (FT) and silty sand (FS).
Eleven samples of 150ml landfill leachate were added into 250ml cleaning Erlenmeyer flasks respectively. After the temperature becomes constant, 5g soil samples FS were added into each Erlenmeyer flasks. In the process of the test, oscillation frequency was controlled at 60rpm, and oscillation periods were 1min, 3min, 8min, 20min, 1 hour, 2 hours, 6 hours, 12 hours, 16 hours, 20 hours and 24 hours respectively. And then, solid and liquid phases were segregated using a centrifuger, and the ammonia-nitrogen contents were measured respectively in order to determine the adsorptive dynamic curves of water-bearing media FS (see Fig.1). The determination of adsorptive dynamic curves for FT is the same as FS. The adsorptive dynamic curves are also presented in Fig.1.
According to the Fig.1, it is concluded the adsorptive dynamic curves of
ammonia-nitrogen are logarithmic. In other words, the adsorption rate decrease
with the increase of time. The adsorption for FT and FS reach equilibrium after
20-24 hours, so 24 hours may be used as equilibrium time for the following
isotherm measurements.
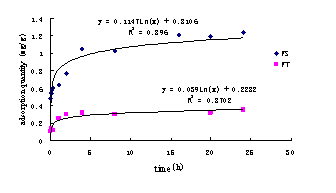
Fig.1 Dynamic curves of ammonia-nitrogen adsorbed by silty soil (FT) and silty sand (FS).
3.4 Determination
of ammonia-nitrogen adsorption isotherms
The landfill leachate mentioned above was diluted to seven different concentrations. Then, 150ml-diluted leachates were added into 250ml Erlenmeyer flasks, and 5g soil (FS) were added. After being oscillated for 24 hours in homothermal oscillator (20℃) at 60rpm, the supernatants were centrifuged for 10min, and ammonia-nitrogen contents were measured. In this case, the equilibrium adsorption quantity can be attained with their mass balance. The adsorption isotherm for FS is presented in Fig.2. The adsorption tests for soil(FT) is the same as the FS.
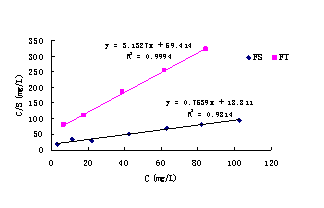
Fig.2 Adsorption isotherms of ammonia-nitrogen for the silty soil (FT) and silty sand (FS).
In the Fig.2, all the adsorption isotherms are Langmuir model despite
the different water-bearing media compositions (organic matter and
clay contents).
4 Retard
Coefficients(R) of Ammonia-Nitrogen
4.1 Determination
of retard
coefficients(R) of ammonia-nitrogen
In order to
simulate the retard action of ammonia-nitrogen adsorbed by the
water-bearing media, the media were installed into soil columns in
accordance with the same bulk density. The adsorption test was conducted with
the landfill leachate under the constant water head. The control conditions are
listed in table 3.
Table 3 Conditions of adsorption test for the water-bearing media
Sample code Column diameter (cm) Column length (cm) water head difference (cm) Hydraulic gradient(-) FS 10 5.5 108 10.8 FT 4 5.5 108 27
Under the condition of constant water head, the leaching tests last for 50 h and 200 h for FT
and FS respectively. The effusive volumes and theirammonia-nitrogen concentrations
were measured periodically. In this case, the curves of the ammonia-nitrogen
concentration related to time can be calculated (Figure 3).
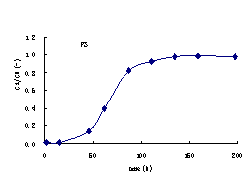
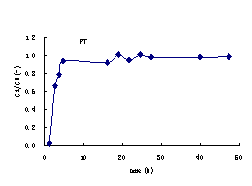
Figure3 Ammonia-nitrogen concentration profile with time
Tab.4 The saturated capability of ammonia-nitrogen adsorbed by the water-bearing media
|
Soil
types |
Cin (mg) |
Cout (mg) |
Saturatedadsorptivecapability, Sm (mg/g) |
|
Silty sand |
482.19 |
210.89 |
0.771 |
|
Silty soil |
293.47 |
201.33 |
0.755 |
4.2 Calculation of retard coefficients(R)
4.2.1 Saturated adsorptive capability of ammonia-nitrogen
(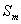 )
)
In order to obtain the
saturated capability of ammonia-nitrogen adsorbed by the
water-bearing media, according to law of conservation of mass, considering the
inlet minus the effluent is equal to capability of ammonia-nitrogen
adsorbed by soil columns at sample interval. The saturated adsorptive capability of ammonia-nitrogen
is calculated as
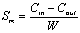 (1)
(1)
thus,
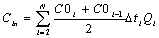
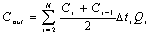
where C0i is
the concentration of ammonia-nitrogen food into soil columns ,
mg/L; Ci is the concentration of ammonia-nitrogen outflow
from soil columns ,mg/L; Qi is the flux outflow
from soil columns, L/d;△ti is the sample interval ,d; N is the
number of sampling period. Cin is the total mass of ammonia-nitrogen food
into soil columns, mg; Cout is the total
mass of ammonia-nitrogen outflow from soil columns, mg/L;W is
the total mass of the water-bearing media in soil columns, g.
The saturated capability of ammonia-nitrogen
adsorbed by two soil columns is calculated from the Eq.(1). The results see
table 4.
4.2.2 Calculation
of retard coefficients(R)
Transport of ammonia-nitrogen in
the soil columns can be approximated as a one-dimensional advection
dispersion equation, here extended with source-sink terms to account for the
sorption process:
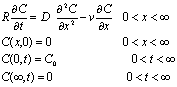 (2)
(2)
where C is the
concentration of the ammonia-nitrogen (mg/L); C0 is
the initial concentration of the ammonia-nitrogen (mg/L); x is
the coordinate in the column(cm); t is the time(h); 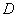 is the
coefficient of hydrodynamic dispersion (cm2);
is the
coefficient of hydrodynamic dispersion (cm2); 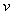 is actual average
velocity of landfill leachate flow in the column (cm/h).
is actual average
velocity of landfill leachate flow in the column (cm/h).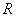 is retard
coefficient(-);Due to the
adsorption isotherms of ammonia-nitrogen are Langmuir model, so
is retard
coefficient(-);Due to the
adsorption isotherms of ammonia-nitrogen are Langmuir model, so
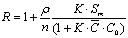 (3)
(3)
here 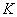 is partition
coefficient of ammonia-nitrogen between solid phase and liquid phase
(cm3/g);
is partition
coefficient of ammonia-nitrogen between solid phase and liquid phase
(cm3/g); 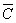 is relative
concentration(-);
is relative
concentration(-);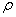 denotes the bulk
density(g/cm3)and
denotes the bulk
density(g/cm3)and 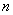 the
porosity(-).
the
porosity(-).
Numerical method
is used to solve Eq.(2). The length of the column are divided into 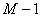 segments,
segments,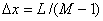 , taking time step
is
, taking time step
is 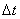 .
. 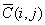 denotes the
relative concentration of where
denotes the
relative concentration of where 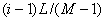 and when
and when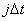 , the following
result of discretization to Eq.(1) used implicit difference method:
, the following
result of discretization to Eq.(1) used implicit difference method:
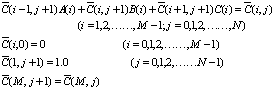 (4)
(4)
where,
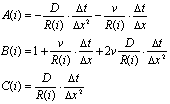
When  of Eq.(3) is
replaced by
of Eq.(3) is
replaced by , it leads to the
, it leads to the  corresponding
expression of Eq.(2) .
corresponding
expression of Eq.(2) .
Due to ,
,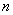 ,
,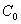 ,
, ,
, ,
,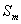 , and
, and 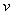 of the
equations are known, selecting the varied value of、 used Langmuir
model lead to series of adsorption curve. By means of comparing
the adsorption curves with the measured breakthrough curves
of the ammonia-nitrogen concentration, a goodness of fit curve
is gained (Fig 4). It shows that it is good fitting to useLangmuir model, corresponding
parameters see table 5.
of the
equations are known, selecting the varied value of、 used Langmuir
model lead to series of adsorption curve. By means of comparing
the adsorption curves with the measured breakthrough curves
of the ammonia-nitrogen concentration, a goodness of fit curve
is gained (Fig 4). It shows that it is good fitting to useLangmuir model, corresponding
parameters see table 5.
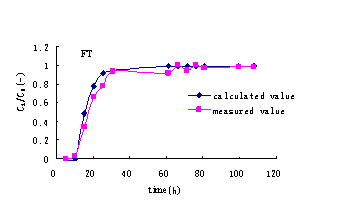
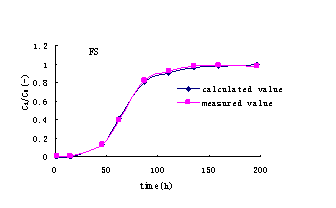
Fig.4 The fitting curve of ammonia-nitrogen concentration profile with time
Table 5 Retard
coefficient and partition coefficient of ammonia-nitrogen at
different water-bearing media
|
Sample |
Flux (cm3/h) |
(cm/h) |
D (cm2) |
K (cm3/g) |
R (-) |
|
FS |
18.71 |
0.79 |
7.70 |
0.10 |
5.00 |
|
FT |
18.45 |
0.73 |
2.28 |
0.12 |
4.85 |
From the table 5, adsorption of the silty sand to ammonia-nitrogen is greater than silt and retard coefficient are 5.00 and 4.85 respectively, which means ammonia-nitrogen of landfill leachate can be adsorbed strongly by the water-bearing media when landfill leachate penetrates into the water-bearing media; Moreover, the value of coefficient obtains from the column experiment is less from absorption isotherms. In theory, the result from column experiment is close to the fact, and is also more dependable.
5 Conclusions
(1) The adsorption
of ammonia-nitrogen on the water-bearing media is conformity
with the logarithmic dynamic curves, which means the adsorption rates
decrease with the increase of experimental time. And the adsorption equilibrium
time is 20-24 hours.
(2)All the
adsorption isotherms of ammonia-nitrogen on the water-bearing
media are Langmuir model although the media have
different adsorptive capacities.
(3) Retard
coefficient is from 4.85 to 5.00, which means ammonia-nitrogen of
landfill leachate can be adsorbed strongly by the water-bearing
media when leachate penetrates into the media; That is to
say,ammonia-nitrogen of landfill leachate not easily move in the water-bearing
media.
Acknowledgement:
This research
work was funded by the Science and Technology Development Foundation
of Tianjin(20040709),and the Startup Scientific Research Foundation
of Tianjin University of Science and Technology.
References
[1] Guo,Y. L.,Wang,Y.
X.,Cai,H.Sh,et al.,2002.Assessment of environment impact of leakage of landfill
site on groundwater. Geological Scienceand Technology Information,21
(1):87-90.
[2] Islam,J., Singhal,
N,2002.A one-dimensional reactive multi-component landfill leachate
transport model. Nironmental Modelling & Software, 17:531–543.
[3] Jiang, H.Zh., Li,
J.P., Zhang,J.,2001.Modeling analysis of leachate pollution of sanitary fill on
underground water in the southern area of Beijing western
suburb.World Geology,20(4):374-378.
[4] Kimmel,G.E.,
Braids,O.C.1980. Leachate plume in groundwater from Babyicm and Islip landfills, Long
Island, New York. Washington,D.C.,US Geological
Survey,(Geological Survey Professional paper 1085).
[5] Liu,Ch.L.,Zhang,Y.,Jiao,P.Ch.,et
al., 2001.The capacity of clayey soil liner at surface layer for
obstructing garbage pollutant in Pudong Area,Shanghai. Acta Geoscientia,Sinica,22(4):360-364.
[6] Ludvigsen, L.,
Albrechtsen,H.J., Heron,G.,et al.,1998. Anaerobic microbial redox processes in
a landfill leachate ontaminated aquifer(Grindsted, Denmark).J.Contam.
Hydrol.33,273-291.
[7] Stumm,W.,
Morgan,J.J.,1996. Aquific chemistry. Wiley, New York.
[8] Wang, H. Q. et
al.,1992. Modeling nitrogen transport and transformation in the unsaturited
zone,Proceedings of
international workshop on groundwater and environment Seismological.
[9] Wang,H.Q., Fei,
J.,1998. Modeling Analysis of Geological Environment Influenced by Landfill.
Bulletin of the Chinese Academy of Geological
Sciences,19:315-324.
[10] Wen,X.L.,Xia, L.J.,
Xu,Y..P., et al.,2004.Effects of leachate on ammonia-nitrogen adsorption
ability in soil nearby a municipal waste landfill. Journal of Agro-Environment
Science, 23(3):503-507.
[11] Xia,L.J., Xu,L.X.,
Wen, X.L.,et
al.,2001.Nitrogen Contamination of Groundwater Originated from Urban Landfill
Leachate. ARro-environrnntal Protection,20:108-110
[12] Zhang,L.H.,Kong,D.F.,Li,R.Q.,2000.Contaminant
resistant mechanism of filled-earth layer in waste disposal site.Chinese
Journal of Geotechnical Engineering, 22(6):678-681.
[13] Zhao,Y.Sh.,Su,Y.M.,Wang,Y.H.,2002.Research
on the landfill site pollintion simulation and control.Environmental
Science,23:83-88.




