Achievements
AN EXPERIMENTAL STUDY OF REMEDIATION OF NITROGEN POLLUTION IN GROUNDWATER WITH PERMEABLE REACTIVE COLUMNS ENHANCED BY MICROORGANISM
Xiao Shangping*,Li Yilian
School of Environmental Studies, China University of Geosciences, Wuhan 430074, China
*Corresponding author.Tel.:+86 027 63111966;
E-mail address: xiaoshangping@126.com
Abstracts: Based on the permeable reactive barriers (PRB) system,
sand filtration (SF) system and constructed rapid infiltration (CRI) system, a
sectional type of permeable reactive column (PRC) was simulated and constructed
in our laboratory to remedy nitrogen pollution in groundwater. Clay, fine sand,
grit and marble were filled in the permeable reactive column in sequence in
order to simulate aeration zone-saturated zone in underground environment.
Microorganism resources were chose from fertile garden mould, the experiment
domesticated and enriched nitrifying bacteria, aerobic denitrifying bacteria,
anaerobic denitrifying bacteria in aerobic condition and anaerobic condition
separately. Then the highly efficient nitrifying bacteria and aerobic
denitrifying bacteria were inoculated into aerobic aeration zone on the top of
the permeable reactive column, the anaerobic denitrifying bacteria which had
good capability were inoculated into anaerobic saturated zone in the substrate
of the permeable reactive column. After running for a period of time, the biofilm in
the permeable reactive column had grown stably, the capability of nitrification
and denitrification were improved highly in this system, the removal
rate of NH4+-N and NO3--N achieved
above 90%. This system was verified to be feasible to remedy nitrogen pollution
in groundwater by modeling study.
Key Words: Microorganism;Permeable Reactive Column;Remedy;groundwater;Nitrogen Pollution
1 Introduction
Nitrogen pollution of
groundwater is a problem recognized throughout the world, many countries and
areas exist this pollution problem with different degrees[1].
Some governments and scholars have attached importance to this problem, the
research of
nitrogen pollution and
control of groundwater has became the hotspot in the world now[2-6].
With the developing of agriculture, the usage of fertilizer has been growing steadily, the contamination of nitrogen in groundwater became more heavy. The soil of unsaturated zone acts as a natural protector to groundwater’ quality, the biological denitrification is one of the most important roles on denitrifying bacteria transform nitrogen in unsaturated zone of soil[7,8], nitrate concentrations in percolating water can be decreased , being reduced to N2O or N2. Today denitrificationis a highly advanced method both technically and scientifically[9-11].
The permeable reactive
barriers (PRB) system is a recently developed in-situ remedy technology for
contaminated groundwater. The concept of PRB is relatively simple. Reactive
material is placed in the subsurface where a plume of contaminated groundwater
flows through under its natural gradient, creating a passive treatment system,
and treated water comes out from the other side after adsorption and reaction
between contaminant and reactive material. After properly designed and
installed, PRB are capable of remedying a number of contaminant with extremely
low maintenance costs[12]. Sand filtration(SF)is the flow of water through a bed of granular media,
normally following settling basins in conventional water treatment trains. The
purpose of this filtration is to remove any particulate matter left over after
flocculation and settling. The filter process operates based on two principles,
mechanical straining and physical adsorption. Sand filtration is a
“physical-chemical process for separating suspended and colloidal impurities
from water by passage through a bed of granular material. Water fills the pores
of the filter medium, and the impurities are adsorbed on the surface of the
grains or trapped in the openings.” The key to this process is the relative
grain size of the filter medium. Constructed Rapid Infiltration(CRI)system is a new type of land
treating technology based on the traditional rapid infiltration(RI)for wastewater[13]. Land treatment system
utilizes natural processes in soils and aquifers to produce high quality water
as opposed to conventional technologies for wastewater reclamation. It is
premised on the infiltration of treated wastewater into the soil and
percolation through the vadosezone. Improvements in water quality can
occur due to many different mechanisms including filtration , biological
degradation , chemical reaction , physical adsorption , ion exchange and
precipitation.
The pollutants of most concern
depending on the groundwater properties were ammonium and nitrate. Then an
efficient nitrogen removal from groundwater should include nitrification and denitrification[14]. Based
on the permeable reactive barriers (PRB) system, sand filtration (SF) system
and constructed rapid infiltration (CRI) system, a sectional type of permeable
reactive column (PRC) was simulated and constructed in our laboratory to remedy
nitrogen pollution in groundwater. Clay, fine sand, grit and marble were filled
in the permeable reactive column in sequence in order to simulate aeration
zone-saturated zone in underground environment. Microorganism resources were
chose from fertile garden mould, the experiment domesticated and enriched
nitrifying bacteria, aerobic denitrifying bacteria, anaerobic denitrifying
bacteria in aerobic condition and anaerobic condition separately. Then the
highly efficient nitrifying bacteria and aerobic denitrifying bacteria were
inoculated into aerobic aeration zone on the top of the permeable reactive
column, the anaerobic denitrifying bacteria which had good capability were
inoculated into anaerobic saturated zone in the substrate of the permeable
reactive column. After running for a period of time, the biofilm in
the permeable reactive column will have grown stably, the capability of
nitrification and denitrification will has been improved highly, this
system will be verified to be feasible to remedy nitrogen pollution in groundwater
by modeling study.
2 Materials and
Methods
2.1 Culture medium
The first culture medium (CM1)
was used for enriching nitrifying bacteria and contained (g/L) (NH4)2SO4 2.0,
K2HPO4 1.0, NaH2PO4 0.25,
MgSO4·7H2O 0.03, MnSO4·4H2O 0.01,
CaCO3 5.0 and distilled water1000mL[15]. The second
culture medium (CM2) was used for enriching traditional denitrifying bacteria
and contained (g/L) KNO3 2.0, K2HPO4 0.5,
MgSO4·7H2O 0.2, C4H4O6KNa·4H2O
20.0, distilled water 1000mL and the pH of this medium was adjusted to 7.2[15].
CM1 and CM2 were the culture medium for enriching, they would induce
nitrifying bacteria and traditional denitrifying bacteria to become the
predominant microorganisms in the biofilm in separate domesticated
container.
The screening medium (SM) was
used to screen and isolate aerobic denitrifying bacteria and contained (g/L)
KNO3 2.0, K2HPO4 0.5, MgSO4·7H2O
0.2, C4H4O6KNa·4H2O 20.0, a few
drops of bromothymol blue (BTB), distilled water 1000mL and the pH of
this medium was adjusted to 7.2. BTB is the critical component in SM, as a pH
indicator, it will turn yellow to blue when pH value changes from 6.2 to 7.6.
It will indicatedenitrification for isolating aerobic denitrifying
bacteria because the pH of SM is between 6.2 and 7.6, and denitrification will
increase the pH of the medium. But it was toxic to bacteria grown on the
culture medium if it was dropped too enough, so we should only drop 1-2 drops
of BTB into the Petri dish for indication.
2.2 Enriching
nitrifying bacteria and traditional denitrifying bacteria
The bacteria resource, aerobic
activated sludge and anaerobic activated sludge were all brought from the Sha Hu sewage
treatment plant in Wuhan. A 2.0 L volume beaker with an aerator
on the bottom was used for enriching nitrifying bacteria from the aerobic
activated sludge in aerobic conditions. A 500 mL volume airproof
plastic bottle with a shaker was used to enrich traditional denitrifying
bacteria from the anaerobic activated sludge in anaerobic conditions.
During the oxidation of
organic material coupling to reduction, oxygen is commonly accepted to be the
first choice as electron acceptor because reduction of oxygen leads to a higher
energy yield than reduction ofnitrate[16]. Therefore, denitrification is
generally thought to occur only under anaerobic condition, which is different
from nitrification occurring under aerobic condition. In this study, we created
aerobic and anaerobic (micro-aerobic) conditions for the growing of nitrifying
bacteria and traditional denitrifying bacteria.
First,
the aerobic activated sludge and anaerobic activated sludge were inoculated
into separate domesticated container according to 10% of effective capacity.
Then the liquid culture medium was added separately after being diluted. With
the pass of time, the dilution multiplier of the added liquid culture medium
changed from 30 to 1, one times was reduced in turn everyday. After a month, we
would improve the concentration of NH4+-N and NO3--N
properly in order to induce nitrifying bacteria and traditional denitrifying
bacteria becoming the predominant microorganism in separate biofilm in
their domesticated container. We changed liquid above the sludge and tested the
removal rate of NH4+-N and NO3--N
everyday.
2.3 Screening and
isolation of aerobic denitrifying bacteria
In the last decades, some
aerobic denitrifying bacteria were reported, such as Thiosphaera Pantotropha, Alcaligenes faecalis, Pseudomonas
nautical, Thaurea mechernichensis, Microvirgula aerodenitrificans and
so on[17-21]. Aerobic denitrification, as a new way to
remove nitrogen from wastewater, makes it possible that denitrification and
nitrification occur at the same time in one reactor, which greatly reduces the
operation cost. There are plenty of aerobic denitrifying bacteria in nature[22].
We thought for screening and
isolating aerobic denitrifying bacteria from nature in order to improve denitrification in
our experimental system. We chose the fertile garden mould brought from a
vegetable plot on the campus of China University of Geosciences as the bacteria
resource. Then the fertile garden mould was washed with 50 mL tap
water and centrifugated, and then the feculent liquid was inoculated into a 2.0
Lsterilized beaker with an aerator on the bottom, and 500 mL liquid
SM was added into the beaker. We changed the liquid SM everyday for the culture
of aerobic denitrifying bacteria in order to cause them becoming the
predominant microorganism in the beaker.
After a certain time, we might
observe some activated sludge growing in the beaker, and with the pass of time,
the activated sludge became more, tinged with gray and downy. Then we
inoculated it onto the Petri dish filled with a layer of sterilized solid
SM, and the Petri dish was kept in the cultivated chest with 30℃, it was favourable to the growing of bacteria colonies on the
solid SM. We chose the blue colony from bacterial colonies in the Petri dish, then purified
it using the dilution-plate technique. The isolated strains were then
tested for their ability to denitrify under aerobic conditions.
2.4 The experiment
enhanced by microorganism inoculation
The lab-scale plant used in
this experimental study consisted of a sectional column (100 cm high and 20
cm diameter) made from polymethyl methacrylate plastics.
Clay, fine sand, grit and marble were filled in the permeable reactive column
in sequence in order to simulate aeration zone-saturated zone in underground
environment.
Then the selected
microorganisms were inoculated, the highly efficient nitrifying bacteria and
aerobic denitrifying bacteria were inoculated into aerobic aeration zone on the
top of the permeable reactive column, the anaerobic denitrifying bacteria which
had good capability were inoculated into anaerobic saturated zone in the
substrate of the permeable reactive column. The column system created a good
nitrification and denitrificationenvironment.
In this study, groundwater
characteristics were the following: NH4+-N 50 mg/L, NO3- -N
50 mg/L, COD (glucose) 1000 mg/L, pH was
adjusted to about 7.0. The permeable reactive column operated with a downward
flow of above groundwater pumped onto the top of the column. After a period of
time, the selected microorganisms attached to different layers in the column
will have been grown more and stably and formed biofilm. We tested the
concentration of NH4+-N and NO3--N
in the outflow from the system everyday for knowing the effect of this system.
3 Results and
Discussion
3.1Nitrification
capability of nitrifying bacteria
Nitrification is the ability
of nitrifying bacteria oxidize NH4+-N to
NO3--N under aerobic conditions, and it is a
oxidation process.
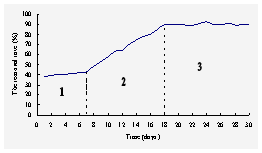
Fig.1 The removal rate of NH4+-N
in the enriching system under aerobic condition
Stage
1 (1-7 days), the aerobic activated sludge had a certain number of nitrifying
bacteria and showed a degreed of nitrification, the removal rate of NH4+-N
was about 40%. Stage 2 (8-18 days), the nitrifying bacteria grown gradually in
the activated sludge and became the predominant microorganism of the biofilm,
meanwhile the removal rate of NH4+-N rose.
Stage 3 (after 18 days), the nitrifying bacteria had become the predominant
microorganism of the biofilm in the beaker, the removal rate of NH4+-N
kept about 90%.
3.2Denitrification
capability of traditional denitrifying bacteria
Denitrification is
the ability of traditional denitrifying bacteria using nitrogen oxides (NO3--N)
as electron acceptors to produce gaseous nitrogen, mainly N2, and it
is a reducing process.
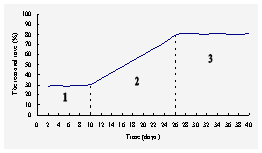
Fig.2 The removal rate of NO3--N
in the enriching system under anaerobic condition
Stage
1 (1-10 days), the anaerobic activated sludge had a certain number of
denitrifying bacteria and showed a degreed of denitrification, the removal
rate of NO3--N was about 30%. Stage 2 (11-26
days), the denitrifying bacteria grown gradually in the activated sludge and
became the predominant microorganism of the biofilm, meanwhile the removal
rate of NO3--N rose gradually. Stage 3
(after 26 days), the denitrifying bacteria had become the predominant
microorganism of the biofilm in the beaker, the removal rate of NO3--N
kept about 80%. Apparently, the denitrifying bacteria grown more slowly
than the nitrifying bacteria.
3.3 Aerobic
denitrifying bacteria isolation
All the isolated and purified
strains from biofilms were tested for denitrifying activity (capacity
of reducing NO3--N to N2O or N2).
Every single isolated strain was inoculated in a sterilized test-tube
with 5-6 mLliquid SM, then each test-tube closed with cotton plugs was
stirred at 120±2 r/min and 30±1℃ on a rotary shaker under
aerobic conditions. After 24 hours, bacteria suspension formed in the
test-tube. Subsequently, 3mL bacteria suspension was transferred into
another sterilized flask containing about 30mL fresh liquid SM. Each flask closed
with cotton plugs was stirred at 120±2 r/min and 30±1℃ on a rotary shaker under aerobic conditions. After 24 hours, we
tested the removal rate of NO3--N for deciding the
denitrifying activity of every single isolated strain.
Table.1 The denitrifying activity of 40
strains isolated from the fertile garden mould sludge
|
Name of strain |
NO3--N removal rate (%) |
Name of strain |
NO3--N removal rate (%) |
|
X1 |
25.6 |
X21 |
63.2 |
|
X2 |
32.9 |
X22 |
19.6 |
|
X3 |
17.8 |
X23 |
25.9 |
|
X4 |
35.8 |
X24 |
32.8 |
|
X5 |
56.8 |
X25 |
48.5 |
|
X6 |
32.8 |
X26 |
37.8 |
|
X7 |
64.7 |
X27 |
24.9 |
|
X8 |
52.3 |
X28 |
19.2 |
|
X9 |
81.6 |
X29 |
28.6 |
|
X10 |
42.6 |
X30 |
52.6 |
|
X11 |
36.6 |
X31 |
17.8 |
|
X12 |
70.6 |
X32 |
22.9 |
|
X13 |
35.4 |
X33 |
24.7 |
|
X14 |
60.3 |
X34 |
42.6 |
|
X15 |
35.6 |
X35 |
38.6 |
|
X16 |
18.9 |
X36 |
62.3 |
|
X17 |
72.9 |
X37 |
56.8 |
|
X18 |
58.6 |
X38 |
49.8 |
|
X19 |
24.8 |
X39 |
26.5 |
|
X20 |
17.4 |
X40 |
69.8 |
The
data showed, the removal rate of NO3--N above 50% had 13
strains, and the assimilation of microorganism wasn’t over 30%[23],
in other words, the 13 strains all had some degree of denitrifying activity
under aerobic condition. The strain X9 had the highest removal rate during
them, 81.6%. Bacterium strain X9 was chosen to be inoculated in the column
owing to its high denitrifying activity.
Then we tested the removal
rate of NO3--N by strain X9 for different concentration
of NO3--N. The strain X9 had a high denitrifying
activity, about 80% removal of NO3--N, while the
concentration of NO3--N was less than 1000mg/L. But when
the concentration of NO3--N was above 1000mg/L, the
removal rate would decline, maybe plenty of NO3--N caused
the NO2--N accumulated owing to denitrification, and
NO2--N was toxic to the microorganisms.
And the bacterium strain X9
will be identified by analysis of the sequence of the encoding 16S rRNA (16S rDNA)
in order to decide whether it is a new one or not and research furtherly.
3.4 The removal of
NH4+-N and NO3--N in the system
enhanced by microorganism
Once the column system had
been inoculated, treatment of the groundwater (NH4+-N 50
mg/L, NO3- -N 50 mg/L) began.
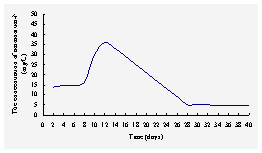
Fig.3 The concentration of NH4+-N
in outlet water from the system enhanced by microorganism
The tranformation of
ammonium ion may involve adsorption, cation exchange, incorporation
into microbial biomass, nitrification or release to the atmosphere in the gas
form. In the first week, adsorption andcation exchange were probably the
major mechanism of removal of NH4+ in this system.,
where positively charged ammonium ion is readily adsorbed onto negatively
charged soil particles, and the concentration ofNH4+-N in
outlet water was about 15mg/L. After a week, the adsorption arrived
saturated, meanwhile the nitrifying bacteria didn’t form a biofilm one
the top layer, in other words, the system had a little nitrification, so that
the concentration of NH4+-N in outlet water
increased and reached a maximum (about 35 mg/L) after 12 days. Then the nitrifying
bacteria became more and the nitrification was notable, the concentration of NH4+-N
in outlet water reduced gradually, and after 28 days, the concentration of NH4+-N
in outlet water was about 5mg/L basically, it went into the stable stage.
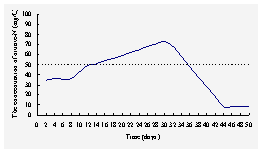
Fig.4 The concentration
of NO3- -N in outlet water from the
system enhanced by microorganism
The
transformation of NO3- -N may involve the
assimilation of microorganism and denitrification in this system. In
the first week, some assimilation maybe the mechanism of removal of NO3- -N,
the concentration of NO3- -N in outlet
water was about 35mg/L. Subsequently, the aerobic denitrifying bacteria and
traditional denitrifying bacteria grown gradually on different layers in the
column, meanwhile the nitrification of the system was stronger than the denitrification so
that the concentration of NO3--N in outlet
water increased and were above 50mg/L soon. After a month, maybe the
aerobic denitrifying bacteria and traditional denitrifying bacteria became more
on their layers, the denitrification of the system became stronger,
the concentration of NO3- -N in outlet
water reduced. After one and half months, the denitrification of the
system went into the stable stage, and the concentration of NO3- -N
in outlet water was about 8 mg/L basically,
4 Conclusions
A major drawback of
groundwater nitrification and denitrification is the fact that
formation of biofilm from its own microbiota in the
influent is necessarily an extremely slow process, owing to the low presence of
microorganisms in the water. And this study presents a possible solution lying
in selective inoculation using bacteria with high nitrifying and denitrifying
activity as inocula, meanwhile carbon source should be added properly.
Based on the permeable
reactive barriers (PRB) system, sand filtration (SF) system and constructed
rapid infiltration (CRI) system, selective inoculation of this permeable
reactive column (PRC) system applied to treat nitrogen pollution of
groundwater is very successful, owing to the inoculated bacteria are the
predominant microorganisms in different layers in the column. In the light of these
results we may state that the capability of nitrification and denitrification were
improved highly in this system, the removal rate of NH4+-N
and NO3--N achieves above 90% so that nitrogen pollution
will be solved basically. This system was verified to be feasible to remedy
nitrogen pollution in groundwater by modeling study.
We should carry out field
experiment to improve this system under different conditions to control a
series of different parameters such as pH, temperature and dissolved oxygen concentration,
because this lab-scale plant was performed in a simple condition.
Acknowledgements
This study was supported by
funds of “2006AA305A01” (the item number),
graduate students’ academic exploration and innovation in China University of
Geosciences (2006), and college students’ scientific research in their spare
time in China University of Geosciences (2006). And it was carried out in the
major laboratory of ministry of education, biogeology and
environmental geology, China University of Geosciences,Wuhan, China.
Our research group, Zhang wei,
Chen huaqing, Yang zhu, Cheng huilin and Zhou xiaojuan are
thanked for their help in the experimental study. Professor Li yilian,
Teacher Luo zhaohui and Professor Liu hui are thanked
for their help and constructive suggestions.
References
[1] Williams A E, Lund L
J, Johanson J A, et al. Natural and Anthropogenic Nitrate
Contamination of Groundwater in a Rural Community, California. Env. Sci .Tech
,1998 ,32(1) :32 -39.
[2] Zhao Lin,Wang Rongshu,Lin Xueyu. Existing
form of nitrogen and its pollution control by microorgnism in the
groundwater. ACTA SCIENTIAE CIRCUMSTANTIAE. July,1999 Vol.19,No.4:443-447.
[3] Hiscock K M ,Lloyd J W,Lerner N.
An engineering solution to the nitrate pollution of borehole at Swaffham ,Norfolk ,U. K.Journal of
Hydrology ,1989 ,107 :267-281.
[4] Andersen L J ,Kristansen H.
Nitrate in groundwater related to landuse in the Karup Basin
,Denmark. Environmental Geology ,1984 ,5 (4) :207-212.
[5] Shen Zhaoli ,Zhong Zuoxin ,Wang
Min et al. Nitrogen pollution and transformation in groundwater system in urban
area. The Proceedings of Scope Workshop on Groundwater Contamination in China.Beijing,1995.
[6] John V. Occurrence and
distribution of pharmaceutical organic compound in groundwater down gradient of
a landfill. Environmental Science and Technology ,1995 ,29 (5)
:1415-1420.
[7] Zhang Sheng,Zhang Cuiyun,Xiao Yu.
Effect Denitrification on Transformation in the Unsaturated Zone of
Soil. Agro-environmental protection. 2002,21(3):254-256.
[8] Wu Yaoguo. Denitrification in
groundwater systems. Techniques and Equipment for Environmental Pollution
Control. Mar.,2002 Vol.3,No.3:27-31.
[9] A.Mohseni-Bandpi, D.J.Elliot,
Groundwater denitrification with alternative carbon sources, Water Sci.Technol.
38 (6) (1998)237-243.
[10] B.O.Mansell, E.D.Schroeder,
Biological denitrification in a continuous flow membrane reactor,
Water Res. 33 (8) (1999) 1845-1850.
[11] B.Moreno, M.A.Go’mez, A.Ramos, J.Gonza’lez-Lo’pez, E.Hontoria,
Influence of inocula over start up of a denitrifying submerged filter
applied to nitrate contaminated groundwater treatment, Journal of Hazardous
Materials B127 (2005) 180-186.
[12] Hu Liming. Permeable
Reactive Barriers for Remedy of Contaminated Groundwater Water. Resources and
Hydropower Engineering. Vol.34 No.7,2003:11-13.
[13] Li Zhengyu,He Tengbing,Pan Caiping,Yang Xiaomao. Apply
in Renovation of Wastewater by Constructed Rapid Infiltration. Journal of
Soil and Water Conservation. Dec.,2004 Vol.18 No.6:41-44.
[14] Li Ping, Zheng Yongliang,
Chen Shuli, et.al. Identification of An Aerobic
Denitrifying Bacterium and Its Potential Application in Wastewater Treatment.
Chin J Appl Environ Biol, 2005,11(5):600-603.
[15] Zheng Ping, Laboratory
Manual for Environmental Microbiology[M] Zhejiang: Zhejiang University Press,
2005.
[16] Frette L, GejlsbjergB, Westermann P.
Aerobic denitrifiers isolated from an alternating active sludge
system. FEMS Microbiol Ecol, 1997, 24:363-370.
[17] Robert son L A, VAN Niel ED
W J, ROB A M. Torremans et al. Simultaneous nitrification and denitrification in
aerobic chemostat cultures of Thiosphaeera Pantotropha. Applied
and Environmental Microbiology, 1988, 54 (11):2812-2818.
[18] Gupta A B. Thiosphaeera Pantotropha a sulphur bacterium
capable of simultaneous heterotrophic nitrification and aerobic denitrification. Enzyme
and Microbile Technology, 1997, 21 (8):589-595.
[19] Chen F, Xia Q, Ju LK.
Aerobic denitrification of Pseudomonas aeruginosa monitored
by online NAD (P) H fluorescence. Appl & Environ M icrobiol,
2003, 69 (11):6715-6722.
[20] Takaya N, Catalan-Sakairi MAB, Sakaguchi Y,
Kato I, Zhou ZM, Shoun H. Aerobic denitrification bacteria
that produced low levels of nitrous oxide. Appl & Environ Microbiol,
2003, 69 (6):3152-3157.
[21] Hu LL, Wang JL, Wen XH, Qian Y.
Improvements on biological nitrogen removal under low dissolved oxygen
concentration. Chin J Appl Environ B iol, 2003, 9 (4):444-447.
[22] Paturead D, Zumstein E, Delgenes JP, Moletta R.
Aerobic denitrifiers isolated from diverse natural and managed
ecosystems. Microb Ecol,2000, 39: 145-152.
[23] Ma Fang, Wang Hongyu,
Zhou Dandan. Selection and enrichment of aerobic denitrifier in
activated sludge system. Journal of Hunan University of
Science & Technology (Natural Science Edition). Jun.,2005, Vol.20,
No.2:80-83.




