Achievements
STUDY ON THE ARSENIC CONTAMINATION OF GROUNDWATER IN THE HETAO PLAIN OF INNER MONGOLIA, CHINA
Cunrong Gao1, Junji Akai2, Xiujun Gao3, Iwao Kobayashi2, Yoshihiro Kubota2,
1
2 Department of Technology and Science,
3 Graduate School of Engineering,
Abstract:To clarify the mechanism of arsenic contamination of groundwater, the Hetao Plain of Inner Mongolia, China, one of the arsenic severely contaminated areas, was studied as a case. To reveal where the arsenic came from and how it was accumulated in groundwater and sediments, this paper discusses the mechanism of migration, accumulation and remigration of the arsenic from geological, geochemical and hydrogeochemical viewpoint. It was found that there are two main causes giving rise to arsenic contamination of groundwater in the Hetao Plain: 1) in natural geological conditions, arsenic in sediments remigrated by groundwater in a long time, and 2) influence of human activities, such as penetration of irrigation water. In the polluted area, arsenic is mainly scattered in the clay and silty clay layers located at less than ac
Key words: groundwater, arsenic contamination, mechanism, the Hetao Plain,
Introduction
Arsenic contamination of groundwater is one of the important environmental issues that threaten public healthy and living level. In recent years, arsenic contamination of groundwater has become a major environmental geological issue all over the world. The high concentration of arsenic in groundwater has been reported not only in many countries of Asia (B.K.Mandal, K.T.Suzuki, 2002) but also in western and south-western US (Davis et al. 1994).
The Hetao Plain, covering a total area of
Since the poisoned patient was first found at Shengfeng
1 Methodology and research process
1.1 Experimental section
1) Samples collected form western and eastern part of the Hetao Plain.
Investigations on drinking water wells (at intervals of 5 –
2) Samples collected from the central part of the plain.
Arsenic, iron and organic(C, N) content were tested for the rock core samples. Arsenic release test, X-ray diffraction analyses and EDS analysis were carried out for some of samples in different media. In a
3) Samples collected from regions around the plain
Arsenic and iron contents in rock core samples were measured by atomic fluorescent spectrometry (WYD-2) and atomic absorption spectrometry (PE3030) at the Inner Mongolia Rock and Mineral Research Institute. X-ray diffraction analyses were carried out by using a Rigaku Geigerflex(Red-X system) at the science department of
1.2 Release test
Samples for the release tests were collected from survey rock cores, surface soil and minerals of the pyrite ore taken at the toe of the Lang Ya Shan Mountains located in the western part of the Hetao plain. The test procedure is as follows:
All the specimens were dried at a low temperature, and crushed into particles less than 200 meshes. In the alkaline solution test
2 Results of investigation and tests
2.1 Investigation results of regional water quality
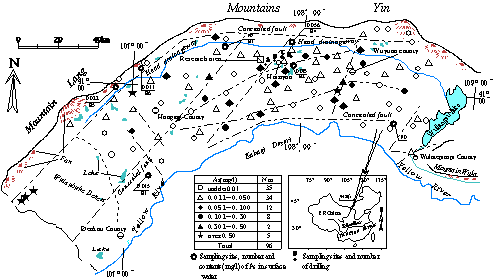
Fig.1 Map of sample location and distribution of arsenic content in groundwater (sample collected from wells at village)
The maximum value is up to 0.943 mg/L. Contaminated groundwater is almost freshwater with salt content of 53.4-1500 mg/L, electric conductivity of 1278-5000μS/cm(
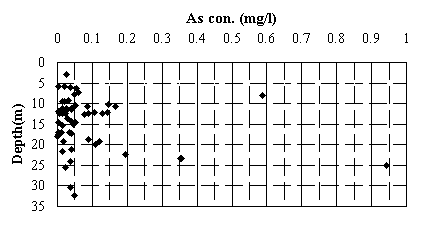
Fig.2 Relation between depth of well and arsenic concentration of groundwater.
(sample locations shown in Fig.1).
Table 1 Arsenic contamination of surface water
Sample location | No. | Concentration(mg/L) |
Irrigation water from | E1 | 0.015 |
E2 | 0.075 | |
Upper reaches of main drainage canal | E3 | Undetected |
Middle reaches of main drainage canal | E4 | 0.056 |
River water coming from western rural area | E5 | 0.011 |
River water coming from western rural area | E6 | 0.022 |
2.2 Results of borehole survey and release test
The analysis results of groundwater samples collected from 4 bore holes (as shown in Fig.1 (A, B, C, D)), including As and Fe concentration of bore-hole soil collected from A, and release test results are given in table 2 and table 3 respectively.
Table 2 As and Fe concentrations and TDS in groundwater at various depths in location A, B, C and D (Gao,1999)
Sample number | Depth | Concentration (mg/L) | ||
As | Fe | TDS | ||
A1 | 17 | 0.072 | 1.490 | 1360 |
A2 | 75 | 0.315 | 3.570 | 10200 |
B1 | 45 | 0.044 | 0.440 | 1860 |
B2 | 54 | 0.129 | 3.660 | 7400 |
C1 | 27 | 0.079 | 0.900 | 650 |
D1 | 19 | 0.016 | 0.310 | 1260 |
D2 | 38 | 0.217 | 0.217 | 1300 |
Table 3. Arsenic levels in samples from borehole soils and minerals and results of elution experiments
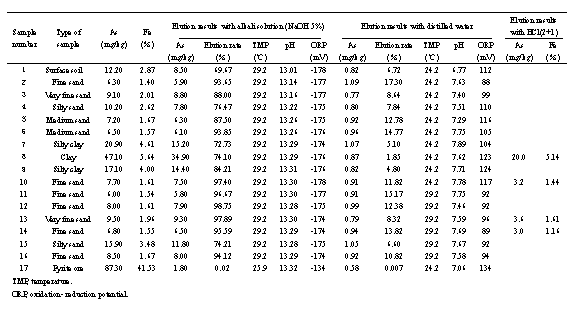
It shows that there are relatively close relations between arsenic and iron concentration and soil characteristics. Arsenic contents range from 10.2 to 47.10mg/kg in clay, silty clay and silty sand, but in silty sand, fine sand and medium fine sand with lower values of 6.3-9.5mg/kg. Iron contents in borehole soils have the same characters as that of arsenic in the above various soils. It shows a positive correlation between iron and arsenic in soils of this area, as shown in Fig.3.
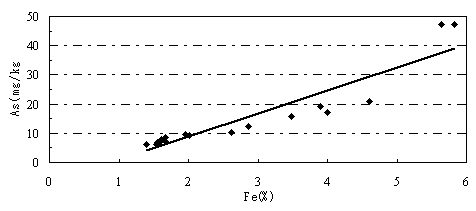
Fig.3 Arsenic and iron contents in borehole soils.
2.3 Survey results of water quality in the study area of the central part of the Hetao plain (Fig.1). Arsenic concentrations of 28(74%) out of 38 wells are above the
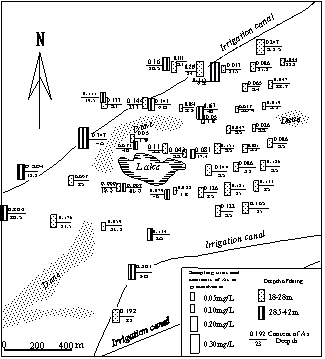
Fig.4 Distribution of arsenic concentration of groundwater at different depths in the investigation area (Gao et.al.,1999).
3 Analysis
3.1 Circulation of arsenic at the earth crust surface
Circulation model of arsenic and regional distribution of arsenic contaminated groundwater are shown in Fig. 7 (Gao, 2000). Arsenic contamination in groundwater caused by natural reason is an environmental hydrogeochemical phenomena occurring in the circulation process of arsenic at the crust surface (Gao, 2000). Primary arsenic is mainly existing in two forms, i.e., as solid existing in primary rock and mineral, or as ion or component existing in deep groundwater (primary water, thermal water and fissure water et al.). The above two forms of arsenic affected by various natural factors and human activities are released into groundwater and directly

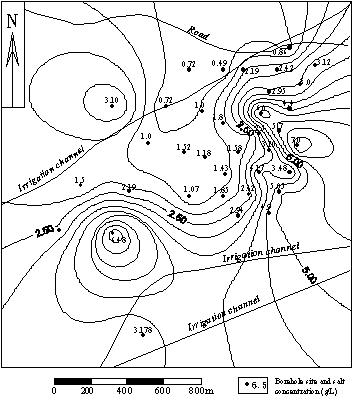
Fig.5 Contour of arsenic concentration of groundwater at depths of 18
lead to groundwater pollution in the following two regions: 1) around ore; 2) along fault or around thermal spring. Arsenic scattered to the earth crust surface due to earth crust movement and various weatheringre-accumulated at the bed of old river, delta and old lake with colloid absorption and mineralization. Over a long period of time and the change of environment, arsenic released from sediment into the groundwater results in much severer groundwater contamination. It is discovered that arsenic contamination of groundwater occurring in many countries of Asia is similar to the contamination mechanism mentioned above(Yamazaki et al. 2000; Research Group for Applied Geology, Sub-group for Arsenic Contamination et al. 2000.
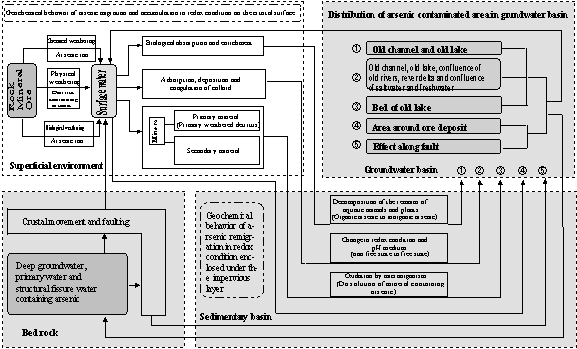
Fig.7 Arsenic circulation model on the crust surface and the distribution of arsenic contaminated groundwater (Gao 2000)
3.2 Mechanism of arsenic contamination in the Hetao Plain
Layers of clay, silty clay and silty sand high in arsenic found from the surface to the depth of more than
layers is from 3 sources. ①Rural region around the plain.
Large pyrite ore rich in arsenic are distributed in the rural region of the western part of the Hetao Plain. The concentration of arsenic measured in mineral is as high as 87.30mg/kg. Arsenic concentration in the surface water from the rural region is 0.011-0.022 mg/L, while in the sediment of surface water reaching 17.6-22.7 mg/L (Gao, 2000), and in rock of this region
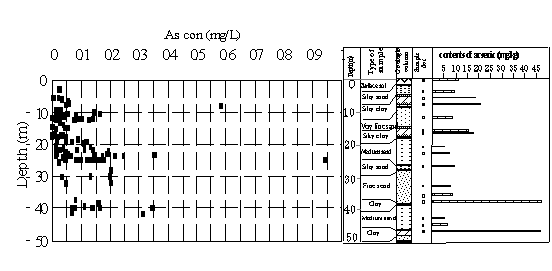
Fig.8 Arsenic concentrations in the groundwater and layers
up to 10-60mg/kg (Li & Li, 1995). Arsenic in rock is 5.5-39 times as high as that of earth crust (1.8ppm) (Oki et al. 1989). It is supposed that rocks and minerals containing arsenic over a long period of weathering and erosion, with impaction of surface water, are accumulated in the sediments of old lakes.
② Upper reaches of the
Most of sediments in the Hetao basin are mobilized by the Yellow River, and arsenic concentration ranges from 13.7 to 17.0mg/kg in modern sediment of the
③ Deep groundwater
The result of borehole investigation shows that at the same site arsenic concentration of the groundwater increases with increasing depth, and that iron and salt concentrations have the same tendency. Tectonic movement was relatively intensive in the Hetao Plain. Deep groundwater rich in arsenic effused out along the fault and arsenic was easily accumulated in the shallow layers. Confined water is alkalized and microelementshows extreme anomaly along tectonic line at the north of Hangjinhouqi, in western part of the plain, between Linhe and Wulamuqianqi, in the southern part of the plain and Shengfengxiang, where arsenic poisoning wasfirst founded, located in the central and eastern part of the Plain (Inner Mongolia Hydrogeological Team, 1982).
Concentrations of B, I and Br in the groundwater of this plain are 1.2-12.0,3.0-80.0 and 18.0-80.0mg/L respectively, while salt content up to
As mentioned above, the layers rich in arsenic are mainly clay, silty clay and silty sand layer (Fig.8). These layers belong to strata of Holocence Series (Q4) and Early Pleistocene Series (Q3) and the sedimentaryenvironment is a transition environment, which is transformed from the reductive environment of deep lake phase to oxidative environment of lake, swamp and river phase (Inner Mongolia Hydrogeological Team, 1981;Sun,1984)
The results of release tests indicate that arsenic in sediment is prone to be dissolved in alkali solution (pH 13.1-13.29) and the release rate is 87.5-98.75% for sand sediment and 72.73-84.21% for clayey sediment. In dilute hydrochloric acid solution (HCl [2+1]) the release rates for the above two kinds of materials are 41.56% and 42.46% respectively. In alkali solution, the release rate of arsenic for pyrite ore is only 0.02%. The test results by X-ray and EDS installed with electric microscope manifest that arsenic minerals are not found in 8 borehole samples collected from different depth and different rocks in the polluted area, including arsenic content reaches 47.1mg/kg in one of samples. Concentrations of organic matter in 18 borehole core samples show that there are no positive correlations between concentration of arsenic and TN+TOC (content of total organic matter in sample, ranging from 0.0409 to 0.6254%). Therefore, it is estimated that arsenic exists in non-crystal compound form or in colloid form. This result is consistent with the analysis results of the form of arsenic existing in sediment and soil (Sakata (1987), Brannon and Patrick (1987), Mok and Wei (1989, 1990), Han et al. (1991)).
The most important mechanism of arsenic accumulation in sediment is that arsenic is adsorbed by iron hydroxides and deposited in the process of interaction between water and sediment (Aggett and O’Brien,1985;Aggett and Roberts, 1986). Concentrations of iron and arsenic show clear correlations in the sediment of the Hetao Plain. As above-mentioned, the Pale-geographic environment in Late Pleistocene is a transition environment from reductive environment to oxidative environment. Acid water rich in organic matter helpful for formation of colloid is easily formed in such environment. Fe(III) in water forms positive colloid of [Fe(OH)3]n or [Fe(OH)3]·Fe3+ and arsenic forms negative colloid. Acid swamp water and mineralized water, containing lots of colloid particles, mixing with the neutral
In addition, as described above, distribution of arsenic contaminated area has good relationships with fault in the Hetao Plain. It is estimated that the concave landform formed from the fault eventually turns into the bed of river. Deep groundwater rich in ion of Fe(Ⅱ) effuses from fault. The Fe(Ⅱ) is oxidized into Fe(III) by surface water and atmospheric oxygen. The Fe(III) adsorbs arsenic of water in the process of mix, coagulation and deposition, finally is accumulated at the bed of river. It is supposed that this is the main reason of contaminated areas distributed in linear.
The reaction process can be represented as the following equations (Munemiya and Tsuno, 1998)
4Fe2+ + 2H2O → 4Fe3+ + 4OH-
2Fe3+ +6OH- → 2Fe(OH)3(=Fe2O3·3H2O)
And (Mok et al., 1988; Wilson and Hawkins, 1978),
Fe(OH)3 + H3AsO4 = FeAsO4·2H2O + H2O
Arsenic release and mobilization is affected by pH, Eh and arsenic concentration of fissure water (Xu and Grimvall, 1988;Mok and Wai, 1989,1990;Brannon and Patrick, 1987;Masscheleyn et al., 1991).
① Oxidation-Reduction condition
With increasing oxidation (decreasing Eh), not only AsO(OH)3 and As(V) in soil are reduced into AsO(OH)and As(III)respectively, but ferric arsenate and Fe(III) combined with other Arsenate are reduced into dissolvable Fe(II) (Hu·Luo, 1984; Shimada,1997). Namely, concentrations of dissolvable arsenic and iron increase with decreasing Eh.
In many areas, sources of arsenic in groundwater are mostly similar, such as silty medium fine sand and silty clay layers (Hu and Lou, 1984; Shimada, 1997; Tonokai and Mitamura 1997). It is supposed that these layers are aquitards, which are covered with Quaternary alluvial clay. Eh nearly shows negative value in such a closed groundwater basin. The reductive environment is helpful for content of dissolvable arsenic rising andfor arsenic mobilizing to water filled in soil pores. Irrigation of the
② Acid and alkali condition
Acid and alkali conditions play important roles on arsenic adsorption in strata. As Eh decreasing and pH increasing, solubility of arsenic increases (Han, 1991). Namely, with Eh decreasing, [Aso4]3- reduced to [Aso3]3- , adsorption of soil particles is reduced. Because of pH increasing, positive electric charges of colloid decrease. Colloid is weakened by the ability of arsenic adsorption and arsenic concentration in soil increases.This result is proved by the release test in alkali solution. As (V) content eluted from sediment increased rapidly when pH values changed from 6.3 to11.5 (Mok and Wai, 1989). When Fe(OH)3 adsorbing arsenic was put into solution with different pH values, content of arsenic dissolved from solution increased with pH value increasing (Kaneko, 1979). PH value (7.2-8.6) in the groundwater of the Hetao Plain is beneficial for the elution of arsenic.
Another factor is the effect on colloid forming in acid water. The pH value in this area is 8.1 for irrigation water and 9.7-9.9 for lake water. The water infiltrated into subsurface causes the colloid forming in acid water decomposed. Thus, arsenic adsorbed by colloid is in a
Fig. 4 and 5 show distributions of groundwater containing high concentration arsenic on the both sides of irrigation drainage in Haiziyan.
Conclusion
Arsenic contamination in groundwater in the Hetao Plain of the
The dominant sources of arsenic are derived from layers of clay, silty clay and silty sand from the surface to the depth of more than
(1)Rural region around the plain, especially with large pyrite ore rich in arsenic in the western part of the plain.
(2)Deep ground water.
(3)Part sediments of the
Reference
[1] Asia Arsenic Network (AAN)(1997) Synthesis in situ investigation report on arsenic contamination in Inner Mongolia -Japan and China, 2-9. (in Japanese)
[2] Aggett J and O’Brien GA (1985) Detailed model for the mobility of arsenic in lacustrine sediments based on measurements in
[3] Aggett J and Roberts LS (1986) Insight into the mechanism of accumulation of arsenic and phosphate in hydro lake sediments by measuring the rate of dissolution with ethylenedaminetetraac aicd. Environ Sci Technol, 20: 183-186.
[4] Brannon JM and Patrick WHJr. (1987) Fixation, transformation, and mobilization of arsenic in sediments. Sci Technol, 21: 450-459.
[5] Fang Hanhui (1984)Outline of Inner Mongolia hydrogeology, Symposium of Geology in Inner Mongolia, 97-98. (in Chinese with English abstract)
[6] Huang Yang-Chu (1994) Arsenic distribution in soils. Nriagu, J.O. (ed) Arsenic in the environment, PartⅠ: Cycling and characterization, 17-49.
[7] The China Research Group of IGCP Project 206 (1989), IGCP Project 206-― Comparison of characteristics of global active faults, Maps of China active faults, Earthquake Press, Xi’an Map Press, Xi’an,30p. (in Chinese)
[8] Gao Cunrong (1997) The geological setting of arsenic contamination in
[9] Gao Cunrong, Toshiaki Ito, Akio Kanai, Shigeru kobayashi ang Hidenori Yokoi (1998) Salinization of soil and arsenic polluted groundwater in the Hetao Plain,
[10] Gao Cunrong and Research Group for Applied Geology, Inner Mongolia Groundwater Research Sub-Group (1999) Arsenic pollution of groundwater in the Hetao Plain of Inner Mongolia, China. Earth Science, 53: 434-451. (in Japanese with English abstract)
[11] Gao Cunrong (1999) Research on the mechanism of arsenic pollution in groundwater in the Hetao Plain,
[12] Gao cunrong (2000) Study on the arsenic pollution of groundwater — A case study in the Hetao Plain of Inner Mongolia, China —. Dr. paper of Graduate School of Science and Technology,
[13] Han Yulian(1991)Soil pollution chemistry, Hong Shuchuen, Chen Yingxin, Han Yulian and Zhang Jingzhen (Ed),Environmental chemistry, East China Normal University Press, Shang Hai, 334-339. (in Chinese)
[14] Hu Shaojie and Luo xin(1984)Primary analysis of arsenic contamination of groundwater in Wu Han city, Department of Hydrological and Engineering Geology (Ed), Problems of environmental hydrogeology (20), Geology Agency Press, 220-233.(in Chinese)
[15] Kanako E, (1979) Arsenic concentration of groundwater in
[17] Masscheleyn PH,
[18] Mok WM, Riley JA, and Wei CM (1988) Arsenic speciation and quality of groundwater in a lead-zinc mine. Water Res, 22: 769-774.
[19] Mok WM and Wei CM (1989) Distribution and mobilization of Arsenic species in the Blackbird Mining area,
[20] Mok WM and Wei CM (1990) Distribution and mobilization of arsenic and antimony species in the
[21] Hygiene Epidemic Prevention Station of Wuyuan Town (1995) Investigation report of local arsenicosis epidemic in Wuyuan, 2-15. (in Chinese)
[22] Hydrogeological team of Inner Mongolia (1982), Research report on hydrogeoligical condition and mitigation approach for pickled soil in the Hetao Plain,
[23] Oki D, Ozhawa L, Tanaka G and Thihara H(Ed)(1989) Chemical Dictionary, Tokyo Chemical Doujin Publishers, 1868p.(in Japanese)
[24] Li Shufan and Li Haoji(1995)Discussion on geo-environmental characters and formation of arsenic contaminated area in the Hetao Plain, Inner Mongolia,Inner Mongolia Geology, No.81・82,6-15.(in Chinese with English abstract)
[25] Sun Wencheng (1984)Primary research on Quarternary geology of the Hetao Plain, Inner Mongolia Geology Society, 274-292.(in Chinese with English abstract)
[26] Shimada I (1997) Geo-environment of groundwater containing arsenic- a case study of northern part of Fukuoka, Minato H and Environmental Geological Research Committee of Journal of Japan Geology (Ed), Series of Geo-environment and Earth Environment 4, Toukai University, 93-114.(in Japanese)
[27] Research Group for Applied Geology, Sub-group for Arsenic Contamination and Miyazaki University Research group for Arsenic Contamination in Groundwater (2000) Arsenic contamination in groundwater and hydrogeological background in
[28] Sakata M (1987) Relationship between adsorption of As(Ⅲ) and boron by soil and soil properties. Environ. Sci. Technol., 21:1126-1130. ok
[29] Munemiya K and Tsuno Y(1998)Basic science for water environment, Korona Publishers, Tokyo, 36p.(in Japanese)
[30] China Remote Sensing Satellite Ground Station,(1997), LANDSAT Pictures (1:50) of the Hetao Plain.(in Chinese)
[31] China Seismological Bureau(Ed)(1989)Satellite pictures of main active faults in China, Science Agency Press, Beijing, 84p.(in Chinese)
[32] Tonokai K and Mitamura M(1997)Arsenic in artesian well and groundwater and effect of geology – Arsenic in artesian well and groundwater and its source in the northern part of Osaka- , Sou Hideo and Environmental Geological Research Committee of Journal of Japan Geology (Ed), Series of Geological and Earth Environment 4, Tougai University, 63-93.(in Japanese)
[33] Wilson FH, and Hawkins DB (1978) Arsenic in streams, stream sediments, and groundwater,
[34] Xu H, Allard B and Grimvall A (1988) Influence of pH and organic substance on the adsorption of As(Ⅴ) on geologic materials. Water, Air, Solt Pollut, 40: 293-305.
[35] Yamazaki C, Ishiga H, Dozen K, Higashi N, Ahmed F, Sampei Y, Md. Hamidur Rahman and Md. Badrul Islam (2000) Geochemical composition of sediments of Ganges delta of Bangladesh -arsenic releasa from the peat ?-. Earth Science, 54: 81-93. (in Japanese with English abstract)
[36] Yang R Y and Ye J (1999) Determination of chemical species of arsenic in environmental water for endemic arsenism area of Bayinmaodao by neutron activation. Pan-asia Pacific Conference on Fluoride and Arsenic Research. Progranm and Abstract Book.




