Achievements
STUDY ON LOCALIZATION OF DEGRADATION ENZYME FOR N-HEXADECANE AND ENZYMATIC DEGRADABILITY
Chen Yan-jun, Wang Hong-qi, Zhang Ping, Yunying
State Key Joint Laboratory of Environmental Simulation and Pollution Control,
Abstract: Petroleum hydrocarbons cause serious environmental problems. However, the mechanism of degradation by microorganism was partially unknown. Localization of degradation enzyme in two bacteria for n-Hexadecane, production and characteristics of n-Hexadecane-degrading enzyme were studied in this paper. The cellular extracts of l bacterium were fractionated into extracellular, periplasmic and cytoplasmic fractions after osmotic shock. The results showed that the main enzyme, in the 1 bacterium, which are cytoplasmic and membrane-associated enzymes, decomposed mainly n-Hexadecane. And most of the enzyme activities (both 100%) in 2 bacterium were detected in the cytoplasmic and extracellular extracts. Moreover, culture conditions have a significant effect on the enzyme activity produced in hexadecane cultures of 1 bacterium and 2 bacterium, and the optimum of the concentrations of hexadecane is 10mg/L.The rhamnolipid(
Keywords: microorganism; enzymatic degradation; n-Hexadecane; osmotic shock
Manufacture, transportation and utilization of petroleum hydrocarbons have brought about frequent occurrences of soil contamination with petroleum. Bioremediation is an innovative technology that depends on the indigenous soil microorganisms to transform or mineralize the organic contaminants. However, because the components of the oil and the processes of the transformation, metabolism and degradation are quite complicated, the mechanism of biodegradation by microorganism was not entirely known. Many enzymes are in either extra-cellular, periplasmic or intra-cellular and may be concerned with the processing of transported contaminants (Kohji Miyazaki, 1997). Therefore, the localization of enzymes is a key step in the degradation of compounds, and localization of many enzymes has been reported, e.g. Zhang Chendong reported the degradation an aromatic sulfur-containing compound mainly locates in cell and on the membrane (Zhang Chendong, 2000). Di-n-butyl phthalate-degrading enzyme was an intra-cellular enzyme (Zeng Feng, 2000). And yet there is little or no information available about the distribution of enzyme in bacteria which can degrade petroleum hydrocarbon. Here we used n-Hexadecane as representative pollutant, and the microorganism was taken from Daqing oil field. Experiments were done in the laboratory to deal with localization of the degradation enzyme in two bacteria for n-Hexadecane. Otherwise, the production and characteristics of n-Hexadecane-degrading enzyme were determined.
Materials and Methods
Chemicals. Hexane(99.9% pure) was obtained from J&K Chemicals LTD. n-Hexadecane(99% pure) was obtained from
Bacterial cultures. Two wild-type strains are capable of metabolizing n-Hexadecane, which were isolated from soils polluted by petroleum. The mineral salts medium (MSM) containing 0.4%Na2HPO4, 0.15%KH2PO4, 0.1%NH4Cl, 0.02%MgSO4 7H2O, 0.0005% iron ammonium citrate, 0.0015%CaCl2, and 1ml of trace elements solution per liter. Cultures were incubated in duplicate in 250ml flasks containing 100ml MSM. Glucose and hexadecane were used individually as sole source of carbon and energy. Flasks were inoculated with 10% inoculums of 1 bacterium, 2 bacterium individually and incubated on a rotary shaker (150rpm) at
Biodegradation studies.
Growth studies. Initial studies to examine biodegradation of n-Hexadecane by 1 bacterium and 2 bacterium were conducted by measuring the bacterial growth in medium under agitation at 150rpm.Inoculations were made in MSM supplemented with 0.01% n-Hexadecane and Glucose individually. Samples were removed at regular intervals and measured by optical density (OD) measurement at 600nm.
Determination of n-Hexadecane mineralization. Hexadecane consumption by 1 bacterium and 2 bacterium was determined indirectly by extracting residual n-Hexadecane in the solvent wash obtained from the accumulation assay. Briefly, bacteria were grown to mid-growth phase in flasks containing n-Hexadecane and harvested by washing with the hexane. The resulting solvent washes were collected, pooled, and used for measuring extra-cellular n-Hexadecane. Hexadecane was extracted twice with 10ml of hexane. The organic phases were collected, dehydrated over anhydrous sodium sulfate, concentrated, and used for GC analysis of n-Hexadecane. Consumption of n-Hexadecane was defined as the difference between the initial mass of n-Hexadecane added to the cultures and the remaining total mass after incubation, both intra- and extra-cellular. The extraction efficiency of n-Hexadecane, determined from the recovery of the internal standard from the sample, was above 80%.
Gas Chromatographic analysis. Hexadecane was quantified by GC using a Varian5.5 gas chromatograph equipped with a flame ionization detector (FID) and HP-SE-54 capillary column (
Localization of degradation enzyme.
Osmotic shock method. Recovery of enzyme was carried out immediately after sampling by the cold osmotic shock method (Park and Lee,1998) as follows. Cells from 20ml culture were harvested by centrifugation at 6000rpm for 10min at
Measurement of enzyme activites. To determine the enzyme activity from crude extract, the reaction mixture contained 2ml extra-cellular extract, periplasmic extract, and cytoplasmic extract individually, 8ml of 0.001% n-Hexadecane in
Optimization of the enzyme production. A large number of enzyme assay procedures have been developed which differ not only in certain conditions of the assay, e.g. rhamnolipid concentration or the carbon source employed, but in the underlying principle of the quantification of the enzyme activity.
Characterization of crude enzyme. The reaction was carried out at various temperatures ranging from
Results and Discussion
Biodegradtion tests.
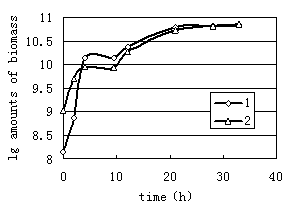
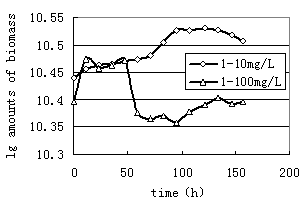
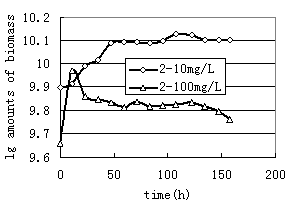
As observed in Fig.1, 1 bacterium and 2 bacterium grown in glucose exhibited stationary growth (as monitored by OD600) over the first 21h. A similar set of experiments were performed with n-Hexadecane as a substrate. The relative growth of bacteria on 10mg/L n-Hexadecane showed a slow, steady increase over a period of 158h(Fig.2, Fig.3). Cell growth entered in the stationary phase after 96h and 47h incubations for 1 and2 bacterium. Thus it is to be noted that both strains utilized n-Hexadecane slowly, while the growth rate of 1 and 2 bacterium in glucose were more rapid. Due to n-Hexadecane extremely hydrophobic nature, it partitions readily into liquid organic phases and exists in aqueous phase only at limited concentrations. This makes uptake of n-Hexadecane by bacteria cells difficult. In bacterial cultures where glucose was dissolved in aqueous solution is utilized easily. The data in Fig.2 and Fig.3 suggest that the rate of two strains growth on 100mg/L n-Hexadecane increased rapidly during the early stages of the experiment (11h), however cells dropped significantly to low levels after 11h. The decreased growth of both strains with increasing n-Hexadecane concentration was consistent with an increasing metabolic load for degradation of the organism. In addition, this is often due to accumulation of the hydrophobic compound in bacterial membranes which can cause devastating effects on membrane structure (Sikkema J, 1994; Sikkema J, 1995 ).
|
|
FIG.1.Growth of 1 bacterium and 2 bacterium grown on glucose | FIG.2. Growth of 1 bacterium grown on 10mg/L and 100mg/L hexadecane |
|
|
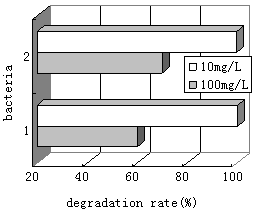
FIG.3. Growth of 2 bacterium grown on 10mg/L and 100mg/L hexadecane | FIG.4. Biodegradation of hexadecane by two strains |
As illustrated in Fig.4, approximately 99% of the 10mg/L initial concentration of n-Hexadecane was degraded within 7d. 1 bacterium and 2 bacterium could take in and decompose 59% and 69% of the originally added 100mg/L n-Hexadecane during this time, respectively. This shows the degradation
capacity of two strains were better. For hexadecane, biodegradation was enhanced at the lower n-Hexadecane concentration (10mg/L). The pattern of n-Hexadecane biodegradation was similar to that of bacterial growth.
Localization of degradation enzyme.
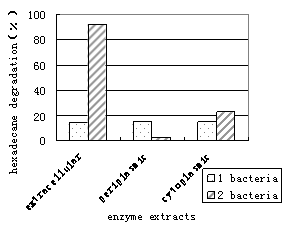
The cellular extracts of bacteria were fractionated into extra-cellular, periplasmic and cytoplasmic fractions after osmotic shock. As illustrated in figure5, the cytoplasmic extract of 1 bacterium is capable of degrading 100% of the n-Hexadecane in 24h. On the other hand, the n-Hexadecane consumption of the membrane-associated and extra-cellular extract were 86% and 47%, respectively. It was revealed that some enzyme proteins residing in the periplasmic space have important functions in the detection and processing of essential nutrients and their transport into the cell (Yuji Nagta, 1999). Therefore, in the 1 bacterium , the main enzymes, which exist in cytoplasmic and membrane-associated extracts, decomposed mainly n-Hexadecane. The enzyme activities of each fraction in 2 bacterium were measured as described previously; most of the enzyme activities (both 100%, Fig.5) were detected in the cytoplasmic and extra-cellular extracts. The enzymes were not detected in the periplasmic space (Fig.5), indicating that enzymes cross both cell membranes without stopping in the periplasm. This result suggests that enzymes are in the periphery of the cells and in the cytoplasmic space.
In contrast, glucose experiments showed that n-Hexadecane was not completely utilized by either extra-cellular, membrane-associated or intra-cellular extracts within the 24h time frame of the experiment (Fig.6). Differences in growth conditions may affect the distribution of enzymes, since we found the lower enzyme activity in glucose-grown cells compared with cell grown on n-Hexadecane.
Fig.5 Distribution of hexadecane-degrading enzyme in the two strains(hexadecane as carbon source) |
Fig.6 Distribution of hexadecane-degrading enzyme in the two strains(glucose as carbon source) |
Optimization of the enzyme production.
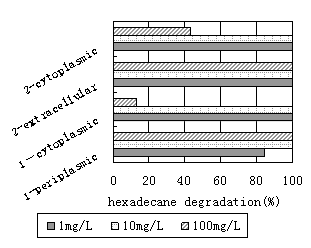
As illustrated in figure7, when both strains grown with hexadecane(10mg/L) as sole source of carbon and energy, extra-cellular, membrane-associated and intra-cellular extracts are capable of degrading 100% of the n-Hexadecane present in less than 24h. The n-Hexadecane consumption of the membrane-associated fraction was less than 85%, when both strains were grown under a rotary shaker with 1mg/L hexadecane as a carbon source. Moreover, the extra-cellular and intra-cellular enzyme activity was not reduced, and the specific
activity of the biodegradation was very high. The enzyme activity was significantly reduced with the higher concentration of hexadecane(100mg/L). As illustrated in figure7, approximately 12.8% and 43% of the n-Hexadecane respectively, were degraded by intracellular extracts of both strains within a period of 24h. We conclude that high concentrations of hexadecane cannot promote the release of in-membrane enzyme by both strains of bacteria, since the growth of the bacteria seemed to be limited. Therefore, the results of this study suggest that culture conditions have a significant effect on the enzyme activity produced in hexadecane cultures of both strains, and the optimum of the concentrations of hexadecane is 10mg/L.
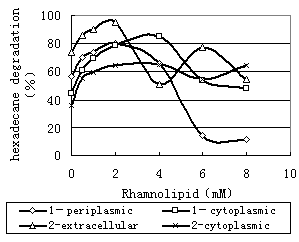
Figure 8 showed that the production of the main enzyme was enhanced at the low rhamnolipid concentration. For 1 bacterium and 2 bacterium, the rhamnolipid (
|
|
Fig.7 Effects of the enzyme production by concentration of hexadecane | Fig.8 Effects of the enzyme production by concentration of rhanmolipid |
|
|
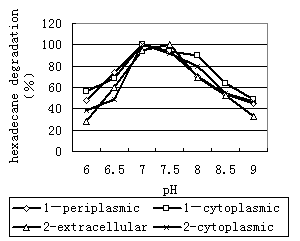
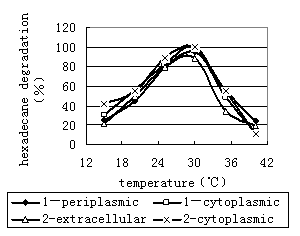
Fig.9 Effects of the enzyme activity by pH | Fig.10 Effects of the enzyme activity by temperature |
Characteristics of n-Hexadecane-degrading enzyme
Effect of pH on the activities of enzyme. The activities of enzyme was quite sensitive to pH(Fig9). The activity of the enzyme was highest between pH 7.0 and pH 7.5. As the pH was increased above 7.5, there was a slight decrease in activity that resulted in a decrease in the degradation rate. On the other hand, as the pH was decreased from 7.0 to 6.0, activity decreased significantly, resulting in an decrease in activity from 100% to <60%.
Effect of temperature on the activities of enzyme. The activity of enzyme was dependent on the temperature during incubation. The activity increases with temperature of incubation until approximately
Thus, the enzyme is active when the pH value is in the range of 7.0~8.0, and the most suitable pH value is near 7.0. The enzyme is active at temperatures up to
Conclusions
This study clearly demonstrated that the main enzymes in the 1 bacterium, which are cytoplasmic and membrane-associated enzymes, decomposed mainly n-Hexadecane. And most of the enzyme activities (both 100%) in 2 bacterium were detected in the cytoplasmic and extra-cellular extracts. Moreover, culture conditions have a significant effect on the enzyme activity produced in hexadecane cultures of 1 bacterium and 2 bacterium, and the optimum of the concentrations of hexadecane is 10mg/L.The rhamnolipid (
Acknowledgments
We thank LIANG Sheng-kang for providing rhamnolipid for assistance in part of experiment work. We would like to thank Xu Jing and her husband for their assistance with revising this paper.
This work was supported by National Natural Science Foundation of China (40472129).
References
[1] A rakane Y, Koga D.1999. Purification and characterization of a novel chitinase isozyme from Yam Tuber. Bio-science Biotechnology Biochemistry. 63 ( 11 ) :1895-1901.
[2] Bouchez M, Blanchet D, 1995. Substrate availability in phenanthrene biodegradation: Transfer mechanism and influence on metabolism[J]. Applied Microbiology and Biotechnology, 43(5): 952-960.
[3] Dibble J T. 1979. Effect of environmental parameters on the biodegradation of oil sludge[J]. Applied and Environmental Microbiology, 37(4):729-739.
[4] Efroymson R A, Alexander M. 1991. Biodegradation by an arthrobacter species of hydrocarbons partitioned into an organic solvent[J]. Applied and Environmental Microbiology, 57(5):1441-1447.
[5] Gomes R C, Semedo L T A S, Soares R M A , et al. 2000. Chitinolytic activity of actinomycetes from a cerradosoil and their potential in biocontrol. Letters in Applied M icrobiology . 30: 146-150.
[6] Kohji Miyazaki. 1997. Degradation and utilization of Xylans by the rumen anaerobe Prevotella bryantii B14. Anaerobe. 3:373-381.
[7] Park S J , Lee S Y. 1998. Efficient recovery of secretory recombinant proteins from protease negative mutant Escherichia coli strains. Biotechnology Techniques. 12 (11), 815-818.
[8] Itoh h. 1971. An assay and screening procedure for serum glutamic oxaloacetic transaminase. Clinical Chemistry .17 (2), 86-88.
[9] Nossal, N. G., and L. A. Heppel. 1966. The release of enzymes by osmoticshock from Escherichia coli in exponential phase. J. Biol. Chem. 241:3055–3062.
[10] Ragher A Al-tahhan. 2000. Rhamnolipid-induced removal of lipopolysaccharide from pseudomonas aeruginosa: effect on cell surface properties and interaction with hydrophobic substrates. Applied and Environmental Microbiology. 66(8):3262-3268.
[11] Sikkema, J., De Bont, J. A. M., & Poolman, B. 1994. Interactions of cyclic hydrocarbons with biological membranes. Journal of Biological Chemistry. 269(11), 8022-8028.
[12] Sikkema, J., De Bont, J. A. M..1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiology and Molecular Biology Review. 59(2), 201-222.
[13] Witholt, B., M. Boekhout, M. Brock, J. Kingma, H. van Heerikhuizen, andL. de Leij. 1976. An efficient and reproducible procedure for the formationof spheroplasts from variously grown Escherichia coli. Anal.Biochem. 74:160–170.
[14] Yuji Nagata. 1999. Two different types of dehalogenases, LinA and LinB, involved in γ-hexachlorocyclohexane degradation in sphingomonas paucimobilis UT26 are localized in the periplasmic space without molecular processing. Journal of Bacteriology. 181(17), 5409-5413.
[15] Zeng Feng. 2000. Study on enzymatic degradability of di-n-butyl phthalate. Chin J Appl Environ Biol. 6(5),477-482.
[16] Zhang Chengdong, Zhang Aiqian. 2000. Localization of degradation enzyme in pseudomonas sp. for an aromatic sulfur-containing compound and identification of products in cells. Environmental Science. 21(4), 90-94.