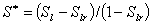Achievements
THE EFFECTS OF CARBON DIOXIDE LEAKAGE ON FRACTURES, AND WATER QUALITY OF POTABLE AQUIFERS DURING GEOLOGICAL SEQUESTRATION OF CO2
Zhang Wei 1, Li Yilian 1,
Xu Tianfu 1, 2, Qiang Wei 1, Xiao Shangping 1
1 School of Environment, China University
of Geosciences, Wuhan, 430074, China
2 Earth Sciences
Division, Lawrence Berkeley National Laboratory, University of California, Berkeley, CA 94720, USA
Abstract:Since industrial
revolution, the “greenhouse effect” is one of the most important global
environmental issues. Of all the greenhouse gases, CO2 is
responsible for about 64% of the enhanced “greenhouse effect”, making it the
target for mitigation, so reducing anthropogenic discharge of carbon dioxide
receives more and more attentions. Geological sequestration of CO2 in deep
saline aquifers is one of the most promising options. But because unknown
fractures and faults may exist in the caprock layers which can prevent the
leakage of CO2, CO2 will leak upward into upper
potable aquifers, and lead to adverse impacts on the shallow potable aquifer.
In order to assess
the potential effect of CO2 leakage from underground storage
reservoirs on fractures and water quality of potable aquifers, this study used
the non-isothermal reactive geochemical transport code TOUGHREACT developed by
Xu et al to establish a simplified 2-D model of CO2 underground
sequestration system, which includes deep saline aquifers, caprock layers, and
shallow potable aquifers, and study and analyze the changes of mineral and
aqueous components.
The simulation
results indicated that the minerals of deep saline aquifers and fractures
should be mainly composed with aluminosilicate and silicate minerals, which not
only increase the mass of CO2 sequestrated by mineral trapping,
but also decrease the porosity and permeability of caprock layers and fractures
to prevent and reduce the CO2 leakage. The results in the deep
saline aquifer found that the mass of carbon dioxide trapped by minerals and
solution phases is limit, the other is remained as a supercritical phase, and
so once the caprock aquifers have some unknown fractures, the free carbon
dioxide phase may leak from CO2 geologic sequestration reservoirs
by buoyancy.
The simulation
results in potable aquifers show that the changes of pH caused by CO2 injection
have significant effects on the water quality in shallow aquifers, especially
the increase of trace metal content caused by the dissolution of metal
minerals. This conclusion not only helps monitor CO2 leakage,
as a purpose of warning,but also the sudden geochemical changes (such as the decrease of pH and
the increase of mineral concentration) in shallow aquifers could be used as
indicators of CO2 leakage from deep geological CO2 repositories.
Keywords: Geological
sequestration; Carbon dioxide; Numerical simulation; Leakage; Fracture; Potable
aquifer; Water quality; Heavy metal
1 Introduction
With the
development of economy and increase of human activities, global emissions of
greenhouse gases (GHG) such as carbon dioxide, methane and nitrous oxides increases rapidly, and leading to the
global warming and climate changes. The “greenhouse effect” has already raised
the Earth’s average temperature and scientists predict that it will climb
gradually by 1–3.58℃ by the year
2100 [1]. Of all greenhouse gases, CO2is responsible
for about 64% of the enhanced “greenhouse effect”, making it the target for
mitigation of greenhouse gases [2]. Atmospheric
concentrations of CO2 have risen from pre-industrial levels of
280 to 358 parts per million by volume (ppmv) in 1994, primarily as a
consequence of fossil fuel combustion for energy production [1]. Fossil fuels,
which nowadays provide 75-85% of the world’s energy [2, 3], will still be
used as a major component of the world’s energy supply for at least the next
century because of their inherent advantages, such as availability, competitive
cost, convenience for transport and storage and abundant
resources.
Therefore, other
ways must be taken to reduce anthropogenic CO2 emissions.
Currently, researches of CO2 geologic sequestration as an effective
way are being carried
out extensively. The main storage formations include depleted
or depleting oil and gas reservoirs, un-mineable coal seams, and deep saline
aquifers [4-9]. Injecting CO2 into
the saline aquifer formation is one of the most promising geologic
sequestration options for the long term because there are regionally extensive
aquifers capable of accepting large volumes of carbon dioxide from power plants
without the need for long transport pipelines [3].
The purpose of
storage CO2 in a saline aquifer can be achieved through
the following four trapping mechanisms [4, 10, 21,
22]: (1) stratigraphic trapping by low permeability capping structures, (2)
solution trapping by dissolution in the formation water, (3) hydrodynamic
trapping by the inhibition of dissolved CO2 due to slow flow of
aquifer fluid, and (4) mineral trapping where CO2 rich
reservoir brine reacts with the reservoir rocks to precipitate carbonate
minerals. Even though a fraction of the CO2 may be trapped in
mineral phases and dissolved into the surrounding water, the bulk mass of
CO2 injected into the subsurface is expected to remain as a
supercritical phase, the free carbon dioxide phase may leak upward into
overlaying potable aquifers through imperfect confinement (e.g. fractures
in the cap rock or abandoned wells) [11, 12]. Dissolution of
CO2 in shallow potable aquifers can cause a decrease in pH.
Such acidic condition can affect the dissolution and sorption mechanisms of
many minerals and groundwater quality by enhancing dissolution and/or
desorption of potentially hazardous trace metals [12]. For example,
galena (PbS) can dissolve and increase significantly Pb concentrations and
diminish groundwater quality in acidic condition [13].
It was assessed
that the potential effect of CO2 leakage from underground
storage reservoirs on fractures (e.g. porosity, permeability and mineral
changes) and water quality of potable aquifers (especially the dissolution of
minerals containing heavy metals) by numerical simulation in this study. Through this research, the geochemical changes could be used
as indicators of CO2 leakage from deep geological CO2 repositories.
This study not only helps the monitoring of CO2 leakage, as a
purpose of warning, but also provides theoretic foundations for the prevention
of CO2 leakage.
2 Modeling Approach
This study uses
the non-isothermal reactive geochemical transport code TOUGHREACT, which
developed by introducing reactive chemistry into the framework of the existing
multiphase fluid and heat flow code TOUGH2, and can be used for the assessment
of mineral alteration in hydrothermal systems, waste disposal sites, acid mine
drainage remediation, contaminant transport, and groundwater quality [15, 17, 26]. Flow and
transport in geologic media are modeled based on space discretization by means
of integral finite differences. An implicit time-weighting scheme is used for
the individual components of the model consisting of flow, transport, and
kinetic geochemical reaction. TOUGHREACT uses a sequential iteration approach,
which solves the transport and the reaction equations separately. The system of
chemical reaction equations is solved by a Newton-Raphson iterative method [14, 15].
The model can be
applied to one-, two-, or three-dimensional porous and fractured media with
physical and chemical heterogeneity. The model can accommodate any number of
chemical species present in the liquid, gas, and solid phases. A wide
range of subsurface thermo-physical-chemical processes is considered. Major
processes for fluid and heat flow are [16, 26]: (1) fluid flow
in both liquid and gas phases under pressure and gravity forces; (2) capillary
pressure effect for the liquid phase; and (3) heat flow by conduction,
convection and diffusion. Transport of aqueous and gaseous species by advection
and molecular diffusion is considered in both liquid and gas phases. Aqueous
chemical complexation, acid-base, cation exchange and gas
dissolution/exsolution are considered under the local equilibrium assumption.
Mineral dissolution and precipitation can be modeled with TOUGHREACT either subject
to local equilibrium or kinetic conditions.
3 Problem Setup
As shown in Fig. 1, this study used
a simplified 2-D model of CO2 underground sequestration system,
which includes deep saline aquifers, caprock layers, and shallow potable
aquifers. On the assumption that the saline aquifer and potable aquifer are
sandstone aquifers and they have the same hydrogeological parameters and
mineral composition. In the present model, the caprock layer was assumed to be
a relatively impermeable shale layer to reduce the impact
of caprock layer on this simulation of CO2 leakage from the
fracture. The model assumed that a fracture was not detected in
geology survey in the caprock layer, through which injected CO2 may
leak upward into shallow potable aquifer. The depth of saline aquifer was
assumed to be 2km, a temperature of 75℃ was used based on a land surface temperature of 15℃ and geothermal gradient of 30℃/km, a pressure of 200bars was used based on
pressure gradient of 100bars/km, the caprock layer and potable aquifer were
assumed according the same principles. In this reactive transport simulation,
hydrogeologic parameters (Table 1), mineral
compositions (Table 2) and total dissolved component
concentrations (Table 3) were cited from the
former researches of Xu et al [13, 15, 17, 18], and modified
appropriately.
In this 2-D model,
the injection well located in the deep saline aquifer and CO2 is
injected through the well at a constant rate of 100kg/s. The injection
rate is approximately equivalent to that of generated by a 300 MW coal-fired
power plant [18]. The CO2 injection was
assumed to continue for a period of 500 years. The fluid flow and geochemical
transport simulation was run for a period of 10,000years.
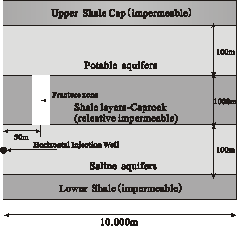
Fig.1 Schematic
diagram for the 2-D model simulation
Table.1 Hydrogeological parameters for 2-D
CO2 injection problem
|
Parameters |
Sandstone |
Fracture |
Shale |
|
Thickness (m) |
100 |
1000 |
100 |
|
Permeability (m2) |
10-13 |
10-11 |
10-28 |
|
Porosity |
0.30 |
0.50 |
0.001 |
|
Compressibility (Pa-1) |
4.5*10-10 |
4.5*10-10 |
4.5*10-10 |
|
Temperature (℃) |
75 (deep saline aquifer) 50 (shallow potable aquifers) |
60 |
60 |
|
Pressure (bar) |
200 (deep saline aquifer) 100 (shallow potable aquifers) |
150 |
150 |
|
Salinity (mass fraction) |
0.06 |
0.06 |
0.06 |
|
CO2 injection rate (kg/s) |
100 (deep saline aquifer) |
|
|
|
Relative permeability: Liquid (van Genuchten, 1980):
|
|
|
|
|
Gas (Corey, 1954):
|
|
|
|
|
Capillary pressure (van Genuchten, 1980):
|
|
|
|
Table.2 List of initial mineral volume
fractions and secondary mineral phases for sandstoneand shale (fault) layers
|
Mineral |
Chemical composition |
Vol.% of solid rock |
|
|
Sandstone |
Shale |
||
|
Saline Potable |
|||
|
Primary |
|
|
|
|
Quartz |
SiO2 |
58.0 58.0 |
19.22 |
|
Kaolinite |
Al2Si2O5(OH)4 |
2.02 2.02 |
4.37 |
|
Calcite |
CaCO3 |
1.93 0.0 |
10.9 |
|
Illite |
K0.6Mg0.25Al1.8(Al0.5Si3.5O10)(OH)2 |
1.0 1.0 |
28.14 |
|
Kerogen-OS |
C64H102O40S10 |
0.0 0.0 |
2.0 |
|
Oligoclase |
CaNa4Al6Si14O40 |
19.8 19.8 |
5.28 |
|
K-feldspar |
KAlSi3O8 |
8.2 8.2 |
4.74 |
|
Na-smectite |
Na0.29Mg0.26Al1.77Si3.97O10(OH)2 |
4.0 4.0 |
23.0 |
|
Chlorite |
Mg2.5Fe2.5Al2Si3O10(OH)8 |
4.55 4.55 |
2.35 |
|
Hematite |
Fe2O3 |
0.5 0.5 |
0.0 |
|
Sphalerite |
ZnS |
0.0 1.0 |
0.0 |
|
Galena |
PbS |
0.0 1.0 |
0.0 |
|
Secondary |
|
|
|
|
Magnesite |
MgCO3 |
0.0 0.0 |
0.0 |
|
Dolomite |
CaMg(CO3)2 |
0.0 0.0 |
0.0 |
|
Low-albite |
NaAlSi3O8 |
0.0 0.0 |
0.0 |
|
Siderite |
FeCO3 |
0.0 0.0 |
0.0 |
|
Ankerite |
CaMg0.3Fe0.7(CO3)2 |
0.0 0.0 |
0.0 |
|
Dawsonite |
NaAlCO3(OH)2 |
0.0 0.0 |
0.0 |
|
Ca-smectite |
Ca0.145Mg0.26Al1.77Si3.97O10(OH)2 |
0.0 0.0 |
0.0 |
|
Pyrite |
FeS2 |
0.0 0.0 |
0.0 |
|
Anhydrite |
CaSO4 |
0.0 0.0 |
0.0 |
|
Alunite |
KAl3(OH)6(SO4)2 |
0.0 0.0 |
0.0 |
Table.3 Initial total dissolved component
concentrations (mol/kg H2O) for reactivetransport simulations in the
underground sequestration system
|
Component |
Sandstone (Saline and Potable aquifers) |
Shale (Fractures) |
|
Ca2+ |
3.23×10-3 |
6.57×10-2 |
|
Mg2+ |
1.53×10-7 |
6.47×10-7 |
|
Na+ |
0.99 |
0.83 |
|
K+ |
7.52×10-3 |
5.6×10-5 |
|
Iron |
2.42×10-5 |
4.92×10-4 |
|
SiO2(aq) |
7.26×10-4 |
5.89×10-4 |
|
Carbon |
4.32×10-2 |
0.92 |
|
Sulfur |
1.32×10-9 |
9.72×10-7 |
|
Al3+ |
2.66×10-8 |
5.41×10-8 |
|
Cl- |
1.0 |
1.0 |
|
Pb2+ |
0.7843×10-11 |
0.7843×10-11 |
|
Zn2+ |
0.6607×10-10 |
0.6607×10-10 |
|
pH |
7.34 |
6.69 |
Note: Iron is the sum of Fe2+,
Fe3+ and their related complexes. Carbon is the sum of CO2 (aq),
CH4 (aq), and their related species such as HCO3- and
acetic acid (aq). Sulfur is the sum of sulfate and sulfide species.
4 Results and
Discussion
4.1 Deep saline aquifers
Although the aim
of this study is to analyze and research leakage problems, the mechanisms for
the storage of CO2 in geological formations must be made
clear primarily.
The simulation results in the deep saline aquifer (Fig.3) shows oligoclase
dissolves completely in both plume and background regions, but the rate of
dissolution in plume region (x<100m) is faster than that in background
region, otherwise the dissolution of chlorite is more significant in plume
region than that in background region. The dissolution of primary
aluminosilicate minerals such as oligoclase, and chlorite provide Ca2+,
Na+, Mg2+, and Fe2+ for the precipitation
of secondary carbonate minerals such as ankerite, and dawsonite in plume region
(Fig.4). The changes of
secondary carbonate minerals are consistent with the changes of CO2 mineral-trapping
capacity during a period of 10,000 years in the deep saline aquifer (Fig.2). So the mass of
CO2 trapped by minerals depends on the dissolution of primary
silicate minerals such as oligoclase, and chlorite and the precipitation of
secondary carbonate minerals such as ankerite, and dawsonite.
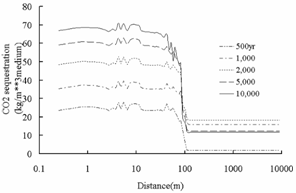
Fig.2 Cumulative CO2 sequestration
by carbonate precipitation for different times.
As Eq. (1) shown, the
injected CO2 can be stored as bicarbonate relative to the
simple solubility of CO2 and is an example of the solution
trapping of CO2 [19]. Similar
reactions can be written for the dissolution of other carbonates.
 (1)
(1)
Oligoclase (CaNa4Al6Si14O40)
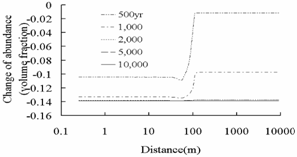
Chlorite (Mg2.5Fe2.5Al2Si3O10(OH)8)
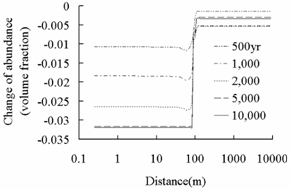
Fig.3 Change of abundance (volume
fraction) of primary aluminosilicate minerals. Positive values express
precipitation and negative values express dissolution.
Ankerite (CaMg0.3Fe0.7(CO3)2)
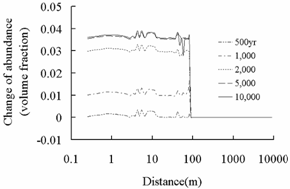
Dawsonite (NaAlCO3(OH)2)
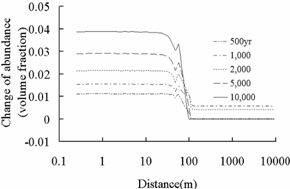
Fig.4 Change of abundance (volume
fraction) of secondary carbonate minerals. Positive value means precipitation
and negative value means dissolution.
The changes of
carbonate (Fig.5) and calcite (Fig.6) are not consistent with Eq. (1) completely,
although the concentration of carbonate after CO2 injection is
much higher than that in the initial dissolved component (4.32×10-2 mol/kgH2O),
the concentration of carbonate does not continue increasing over time, the
reason may be that carbonate in this model is the sum of CO2 (aq),
CH4 (aq), and related species such as HCO3- and
acetic acid (aq).
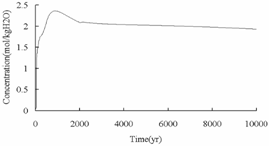
Fig.5 Concentration of carbonate over times
in deep saline aquifers.
In this study, the
simulation results can prove that a fraction of CO2 can
be trapped in mineral and solution phases, but the bulk mass of CO2 injected
into the subsurface is expected to remain as a supercritical phase, the free
carbon dioxide phase may leak from CO2 geologic sequestration
formations by buoyancy, so the evaluation of security is very important to CO2 underground
sequestration.
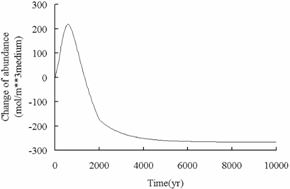
Fig.6 Change of abundance of calcite over
time in deep saline aquifers.
4.2 Fracture
As mentioned
above, the supercritical carbon dioxide is pushed up to the bottom of caprock
by buoyancy, but the action of capillary force makes carbon dioxide difficult
enter into the caprock [19]. But once there
are some unknown fractures or faults of high permeability in caprock layers. CO2 will
leak upward from the "fast-track" to upper aquifers or ground
surface, which may pose potential risks on the environment.
In this study, one
of the most concerns is the effect of CO2 leakage on the change
trend of porosity and permeability in fracture of high permeability. The
relevant research findings can also be used to infer the impact of CO2 injected
on the caprock of low permeability.
Fig.7 shows the
decrease of porosity and permeability over time in the fracture. This
phenomenon may be caused by the precipitation of carbonate minerals, which is
consistent with CO2 mineral sequestration shown in Fig.8. The similar
result was obtained in the deep aquifer reservoir.
As shown in Fig.8, the mass of CO2 sequestrated
by carbonate mineral precipitation in fracture is far less than that in deep
saline aquifers. Through the comparison of secondary mineral abundance changes
in Fig.4and 9, it was found that the
differences between the fracture and deep saline aquifer are consistent with
that in Fig.8. The study above in deep saline aquifers
found a close relation between primary mineral precipitation and secondary
mineral dissolution, precipitation of dawsonite (NaAlCO3(OH)2), requires Na+ provided
by oligoclase (CaNa4Al6Si14O40)
dissolution, and precipitation of ankerite (CaMg0.3Fe0.7(CO3)2)
requires Ca2+provided by oligoclase dissolution mostly and some by
calcite dissolution, Fe2+ and Mg2+ supplied by chlorite
(Mg2.5Fe2.5Al2Si3O10(OH)8)
reduction. The initial mineral volume fraction of oligoclse and chlorite for
sandstone layers (deep saline aquifers) is far more than that for shale layers
(fractures) (Table 2). So in the model, the CO2 mineral
trapping capacity mainly depends on the volume fraction of oligoclase and
chlorite.
It has also been
reported that protons (H+) in aqueous solution will interact with
aluminosilicate minerals (e.g. feldspars, zeolites and clay minerals) releasing
ions such as Ca, Mg, and Fe which can permanently fix CO2 as
the carbonate minerals, and Fyfe et al found that silicate minerals such as
olivine could be sources of Ca and Mg [1, 20]. This is the
reason why siliciclastic aquifers are considered better candidates for CO2sequestration
than carbonate aquifers which will dissolve with CO2 injected [1]. This simulation
results also confirmed the above conclusions, which provide a
theoretical basis for the selection of CO2 underground
reservoirs. Not only the reservoirs, but also the mineral components of upper
caprock should be mainly composed with aluminosilicate and silicate minerals,
which can enhance the mass of CO2 sequestrated by minerals and
reduce the possibility of CO2 leakage from caprock by the
decrease of porosity and permeability to ensure the safety of underground
disposal projects of CO2.
Permeability
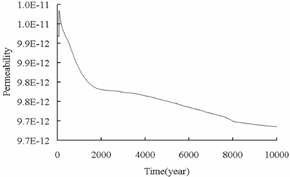
Porosity
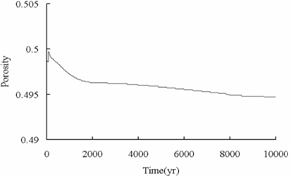
Fig.7 Permeability
and porosity vs. time in the fracture.
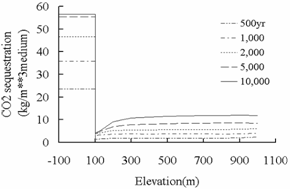
Fig.8 Cumulative CO2 sequestrated
by carbonate mineral precipitation in deep saline aquifer and fracture.
4.3 Potable
aquifers
During the process
of CO2 geological disposal, risk assessment is very important.
One of the most concerns is the potential influences of CO2 leakage
on the geochemical characteristics and quality of upper potable groundwater
including pH, metal minerals, and so on.
Dawsonite
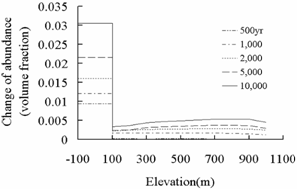
Ankerite
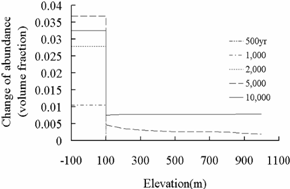
Fig.9 Change of mineral abundance of
dawsonite and ankerite in deep saline aquifer and fracture.
In this study, to study the effects of CO2 leakage on
groundwater quality, the simulation assumed Pb and Zn bearing minerals, galena
and sphalerite, which present in the rock with 1% volume fraction each (Table2
and 3).
As Eq. (2), (3),
(4) and (5) shown [23], with the leakage
of injected CO2 through fracture in the upper caprock, CO2 is
dissolved in water, and forms H2CO3 (aq), HCO3-,
and CO32-, which will result in a decrease of pH [12]. In the
simulation results (Fig.10), the lowest pH is about 5, and with
minerals dissolved, pH can be buffered to about 6, which is below the value of
pH in “Water Quality Standard for Drinking Water” (GB5749-85) promulgated by
China. However, the pH of shallow potable aquifer before CO2 leakage
conform to the standard (Table 3), so the leakage
of injected CO2 has a adverse influence on the changes of pH in
potable aquifers.
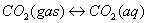 (2)
(2)
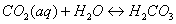 (3)
(3)
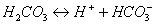 (4)
(4)
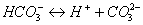 (5)
(5)
Fig.11 and 12 illustrate
the changes of Zn and Pb concentration respectively, solid line in Fig.11 and 12 is the
primary standard of “Water Quality Standard for Groundwater”
(GB/T14848-1993), and the concentration changes have the same trend. Pb poses a
significant public health threat through long term internal accumulation that
can result in damages to the blood system, nervous system, digestive system and
kidney, especially to young children and pregnant women [12, 24], so this paper
studied and analyzed the changes of Pb concentration in order to determine the
effect of CO2 leakage on the concentration of heavy metal ions
in potable aquifers. This study mainly considers the potential impact of
dissolved galena on Pb concentration, because galena is one of the most
important base metal sulfide minerals and one of the main minerals controlling
the mobility of lead in the subsurface [12].
As Fig.11 (a) shown, the
study found that the concentrations of Pb don’t exceed the primary standard of “Water
Quality Standard for Groundwater” (GB/T14848-1993)(0.005mg/L≈2.413×10-8mol/kgH2O)
and the action level (7.843×10-12mol/kgH2O) of Pb under
the Safe Drinking Water Act in 1991 in America. [12]. But comparing
with the concentration of Pb before CO2 leakage (2.413×10-8mol/kgH2O,
Table 3) and the changes of Pb concentration in background region (Fig.11 (b)), the
concentration of Pb in plume region has increased by the leakage of CO2 injected.
The reason why the changes of Pb concentration are not insignificant is the
effect of pH on the dissolution of metal minerals, Fig.10 and 11 (a) shown that
when injected CO2 leak into potable aquifer, the pH decreases
to about 5 and the concentration of Pb increases significantly; but the buffer
role of minerals dissolved on pH will reduce the concentration of Pb. Xu [13] and Wang et al [12] found in
their simulation research that when pH less than 5, galena can be dissolved
significantly, the concentration of Pb increases remarkably and exceeds the
water quality standards; but when pH is higher than 5, the dissolved Pb
concentration will be lower, which are similar with our simulation results.
CO2 used
for geological disposal usually comes from the combustion of fossil fuel, in
which contains a few of acid gases such as H2S and SO2 [25, 27]. Although CO2 should
be captured by physical and chemical methods before CO2 injection,
but it can not be removed completely; once the acid gases are injected with CO2 into
underground reservoirs, it will inevitably cause the marked decrease of pH and
the dissolution of metal mineral, and have adverse impacts on the water quality
of potable aquifers. The future simulations and researches should be focus on
the injection of acid gases such as H2S and SO2.
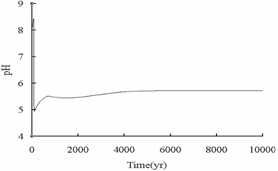
Fig.10 Changes of pH over times in shallow potable aquifers
Pb
(a)

(b)
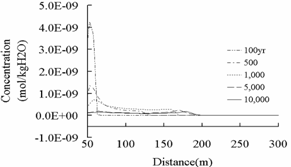
Fig.11 Concentration of Pb in shallow
potable aquifers. (a) Solid line is the primary standard of “Water Quality
Standard for Groundwater” (GB/T14848-1993)(0.005mg/L ≈ 2.413×10-8mol/kgH2O);(b)The distance exceed200m is the background region in
which the leakage of CO2 from deep saline aquifers does not
exist.
Zn
(a)
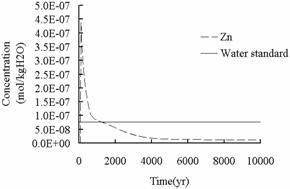
(b)
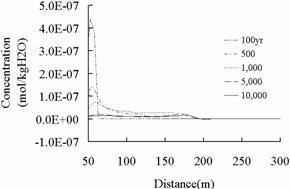
Fig.12 Concentration of Zn in shallow
potable aquifers.
5 Conclusions
In this study, the
non-isothermal reactive geochemical transport code TOUGHREACT was used to
establish a 2-D model and research the impacts of CO2 leakage
from underground reservoir on fractures and potable aquifers.
The simulation
results indicated that the minerals of deep saline aquifers and
fractures should be mainly composed of aluminosilicate and silicate minerals,
which not only increase the mass of CO2 sequestrated by mineral
trapping, but also decrease the porosity and permeability of caprock layers and
fractures to prevent or reduce the probability of CO2 leakage.
The other emphasis
of study is to evaluate the latent influences of CO2 leakage on
geochemical changes and water quality in potable aquifers, especially the
dissolution of metal mineral. The simulation results reveal that the
concentrations of Pb in potable aquifer are lower than corresponding water
quality standard, but the leakage of injected CO2 can lead to
the dissolution of galena and the increase of Pb concentration. Through the
researches and analyses of the results above and other simulation results (Xu, and Wang et al),
it was found that the changes of pH caused by the leakage of injected CO2 play
a vital role in the dissolution of metal minerals and the increase of metal
concentrations. So the geochemical changes in shallow aquifers (such as the
decrease of pH and the increase of mineral concentration) suddenly could be
used as indicators of CO2leakage from deep geological CO2 repositories.
In this paper, as a result of the lack of relevant dynamics data and the limit simulation capability of code TOUGHREACT, current 2D model established in this study didn’t consider the aqueous complexation and oxidation, and the rock adsorption. The future simulation studies would supplement and improve current TOUGHREAR model through laboratory test and related data collection. The numerical simulation software modified will be used to accurately simulate the relevant problems of geological sequestration of carbon dioxide.
Acknowledgements
We thank Wu ChenXi for reviews of the manuscript. This work was supported by NSFC project (No. 40472122).
References
[1] Thomas Gentzis.
Subsurface sequestration of carbon dioxide-an overview from an Alberta Canada
perspective. International Journal of Coal Geology. 43 (2000) 287–305
[2] S. Bachu., J.J.
Adams. Sequestration of CO2 in geological media in
response to climate change: capacity of deep saline aquifers to sequester CO2 in solution.
Energy Conversion and Management. 44 (2003) 3151-3175
[3] D.E. Allen, B.R.
Strazisar, Y. Soong, S.W. Hedges. Modeling carbon dioxide sequestration in
saline aquifers: Significance of elevated pressures and salinities. Fuel
Processing Technology. 86 (2005) 1569-1580
[4] Bachu, S., Cunter,
W.D., Pekins, E.P. Aquifer disposal of CO2: hydrodynamic and mineral
trapping. Energy Convers. Manage. 35(1994): 269–279.
[5] S. Holloway. An
overview of the underground disposal of carbon dioxide. Energy Convers. Mgmt.
1997; 38 (SS), S193–S198.
[6] S. Holloway.
Safety of the underground disposal of carbon dioxide. Energy Convers. Mgmt.
1997; 38 (SS), S241–S245.
[7] S. Holloway.
Storage of fossil fuel-derived carbon dioxide beneath the surface of the earth.
Annu Rev Energy Environ. 2001; 26:145–66.
[8] P. Freund, W.G.
Ormerod. Progress toward storage of carbon dioxide. Energy Convers. Mgmt. 1997.
Vol. 38, Suppl., pp. S199–S204.
[9] Brian Hitchon,
W.D. Gunter, Thomas Gentzis, et al. Sedimentary basins and greenhouse gases: a
serendipitous association. Energy Convers. Mgmt. 40 (1999): 825–843.
[10] S.P. White,T, R.G.
Allis, J. Moore, T. Chidsey, C. Morgan, W. Gwynn, M. Adams. Simulation of
reactive transport of injected CO2 on the Colorado Plateau,
Utah, USA. Chemical Geology. 217 (2005) 387– 405
[11] K. Pruess, J.
Garcia. Multiphase flow dynamics during CO2 disposal in
aquifers. Environ. Geol. 42 (2003) 282–295.
[12] Sookyun Wang,
Peter R. Jaffe. Dissolution of a mineral phase in potable aquifers due to CO2 releases
from deep formations; effect of dissolution kinetics. Energy Conversion and
Management. 45 (2004) 2833-2848
[13] Tianfu Xu.
Importance of mineralogical data for groundwater quality affected by CO2 leakage
from storage sites. PROCEEDINGS, CO2SC Symposium 2006. Lawrence Berkeley Nation
Laboratory, Berkeley,California, March 20-22, 2006
[14] Norifumi Todaka,
Chitoshi Akasaka, Tianfu Xu, Karsten Pruess. Reactive geothermal transport
simulations to study the formation mechanism of an impermeable barrier between
acidic and neutral fluid zones in the Onikobe Geothermal Field, Japan. JOURNAL
OF GEOPHYSICAL RESEARCH. VOL. 109, B05209, doi: 10.1029/2003JB002792, 2004
[15] Tianfu Xu, John A.
Apps, Karsten Pruess. Numerical simulation of CO2 disposal by
mineral trapping in deep aquifers. Applied Geochemistry. 19 (2004) 917–936
[16] Tianfu Xu, Yvette
Ontoy, Phil Molling, Nicolas Spycher, Mauro Parini, Karsten Pruess. Reactive
transport modeling of injection well scaling and acidizing at Tiwi field,
Philippines. Geothermics. 33 (2004) 477–491
[17] Tianfu Xu, John A.
Apps, Karsten Pruess. Mineral sequestration of carbon dioxide in a
sandstone–shale system. Chemical Geology. 217 (2005) 295– 318
[18] Tianfu Xu, Eric
Sonnenthal, Nicolas Spycher, Karsten Pruess. TOUGHREACT-A simulation program
for non-isothermal multiphase reactive geochemical transport in variably
saturated geologic media: Applications to geothermal injectivity and CO2 geological
sequestration. Computers & Geosciences. 32 (2006) 145-165
[19] Robert J.
Rosenbauer, Tamer Koksalan, James L. Palandri. Experimental investigation of
CO2–brine–rock interactions at elevated temperature and pressure: Implications
for CO2 sequestration in deep-saline aquifers. Fuel Processing
Technology. 86 (2005) 1581– 1597
[20] Gunter, W.D. et
al. Technical and economic feasibility of CO2 diposal in
aquifers within the Alberta Sedimentary Basin, Canade. Energy
Convers. Manage. 37(1996): 1135–1142.
[21] Daniel E. Giammar,
Robert G. Bruant Jr., Catherine A. Peters. Forsterite dissolution and magnesite
precipitation at conditions brelevant for deep saline aquifer storage and
sequestration of carbon dioxide. Chemical Geology. 217 (2005) 257– 276
[22] Bruant Jr., R.G.,
Celia, M.A., Guswa, A.J., Peters, C.A., 2002. Safe storage of CO2 in deep
saline aquifers. Environ. Sci. Technol. 36(11), 240A–245A.
[23] Y. Soong, A.L.
Goodman, J.R. McCarthy-Jones, J.P. Baltrus. Experimental and simulation studies
on mineral trapping of CO2 with brine. Energy Conversion
and Management. 45 (2004) 1845–1859
[24] Meng Ziqiang.
Environmental toxicology. Beijing: China Environmental Science
Press. 2000.8 128-134
[25] Xu, T, J.A. Apps,
and K. Pruess, Mineral alteration due to injection of CO2, H2S
and SO2 in deep arkosic formations, in Water Rock
Interaction (WRI-11) edited by Richard B. Wanty & Robert R. Seal, p.
601-605, A.A. Balkema, London, 2004.
[26] Tianfu, Xu, Eric
Sonnenthal, Nicolas Spycher and Karsten Pruess. TOUGHREACT User’s Guide: A
Simulation Program for Non-isothermal Multiphase Reactive Geochemical Transport
in Variably Saturated Geological Media. Lawrence Berkeley Laboratory
Report-55460, Berkeley, California.
[27] W.D. Gunter, E.H.
Perkins, Ian Hutcheon. Aquifer diposal of acid gases: modeling of water-rock
reactions for trapping of acid wastes. Applied Geochemistry. 15 (2000)
1085-1095





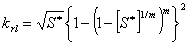
 : irreducible water
saturation
: irreducible water
saturation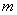 : exponent
: exponent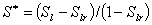
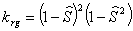
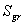 : irreducible gas saturation
: irreducible gas saturation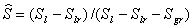
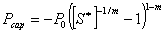
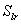 : irreducible water
saturation
: irreducible water
saturation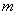 : exponent
: exponent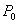 : strength coefficient
: strength coefficient