Achievements
EXPERIMENTAL STUDY ON TRANSPORT LAW OF F- IN SOILS
Zhang Hong-mei ,Su Bao-yu
Abstract: Chemicals’ transport in soils can be influenced by some soil factors, such as soil moisture, texture, homogeneity ,soil temperature ,characteristic of chemicals and the way of input solute and so on .The influence of the texture and the way of input solute on chemicals’ transport under the laboratorial conditions were studied in this paper. The result was that the value of the hydraulic dispersion coefficient and the retardation factor in soil (sand and kaoline mix) was greater than that in sand .In the way of input solute , sodium chloride in solution influenced transport rule of F- in different soils.
Key word: fluorine; transport rule; numerical simulation
Fluorine is widely distributed in nature, it is the necessary element for human beings and animal health. Drinking water with certain concentration of fluoride can prevent dental caries and enhance the bone and the teeth, but drinking water with excessive fluoride in the long run may result in dental and skeletal fluorosis. The problem of fluoride pollution has already been paid more and more attention at present. With the development of aluminium metallurgy ,phosphate fertilizer-making and production of organic fluoride, a great deal of fluoride were let into air, water and soil. In order to realize incidence of fluoride pollution and transport characteristic, we must understand transport rule and dynamic characteristic on fluoride pollution in porous media. This is a key problem for forecasting and controlling fluoride pollution migration. Many factors can affect migration of fluoride pollutant, for example, the process and rule of migration differ on same solute under various condition. The effects on F- migration rule in different soil and input solute are discussed in this paper.
According to subordinate item about study on rule of transportation and transformation for typical solute in soil and groundwater , which is from task about research on ecology geochemistry of economic zone in loess tableland basin, experimental study on mechanism of migration and transformation about fluoride pollutant in laboratory is performed in this paper. In order to be consistent with soils of studied area, the test soils are composed of kaoline and mid-coarse sand according to 1:8 and 1:10. the gradation of test soil is 0.005~
with test soil by layer and the soil height of per layer is
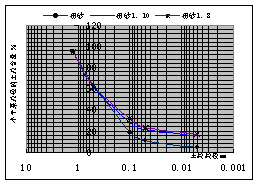
Fig.1 Grain size distribution of different soils
Table 1 Chemical composition of test soil
土样 化学成分 | 高岭土中粗砂(1:10) |
PH | 7.5 |
SiO2(%) | 93.75 |
Al2O3(%) | 3.7 |
Fe2O3(%) | 0.057 |
Ca(mg/kg) | 1.93 |
Na(mg/kg) | 0.82 |
Fe(mg/kg) | 4.54 |
Mn(mg/kg) | 57.4 |
Breakthrough curve is defined as concentration relative of effluent in the bottom of earth column varies with pore volume(BTC).BTC may reflect mixture metathesis and transport characteristic of solute in porous media. The transport parameter of F- can be obtained from fitting breakthrough curve of Cl- and F- in earth column by using CXTFIT program , which is compiled by using least square method according to analytic solution of mathematic controlling equation for chemical substance transport in soil. The program involves chemical property of solute and physical chemistry characteristic of soils, including linear and non-linear equilibrium adsorption model under the condition of equilibrium adsorption, two site and two region model which is considered different sorption rule of soil particle in differ site , stochastic model which is considered soil spacial property. Since chemistry property of Cl- is not active, and negative ion ,the linear mathematical model is used to fit breakthrough curve under condition of considering no source and sink in this paper. Supposing that no sorption and attenuation reaction happen during transport of Cl- ,that is to say, retardation factor Rd=1. The dispersion coefficient D is obtained when velocity of flow u and retardation factor Rd are constants. Retardation factor Rd can be obtained when dispersion coefficient D is obtained. Then, linear mathematical controlling equation is written as
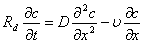 (1)
(1)
Initial condition and boundary condition are expressed by
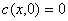 t=0(2)
t=0(2)
![]()
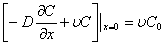 ,0<t≤t0
,0<t≤t0
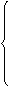
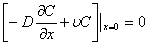 , t>t0 (3)
, t>t0 (3)
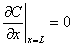 , t>0 (4)
, t>0 (4)
Where 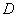 is coefficient of hydrodynamic dispersion,
is coefficient of hydrodynamic dispersion, 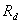 is retardation factor;
is retardation factor; is average velocity of pore water;
is average velocity of pore water;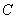 is rudimental concentration of soil;
is rudimental concentration of soil;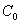 is initial concentration of solute ;L is the height of earth column;t0 is the time of injecting solute;
is initial concentration of solute ;L is the height of earth column;t0 is the time of injecting solute;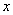 is distance. Transport parameters in three soils see table 2 by fitting breakthrough curve.
is distance. Transport parameters in three soils see table 2 by fitting breakthrough curve.
Table 2 Calculated parameters of different soils
soil parameter | mid-coarse sand | mid-coarsesand and kaoline mix (1:10) | mid-coarse sand and kaoline mix (1:8) |
velocityv(cm.h-1) | 5.5 | 3.6 | 3.85 |
coefficient of hydrodynamic dispersionD(cm2.h-1) | 12.8 | 16.8 | 19.2 |
retardation factorRd | 1.34 | 1.37 | 1.40 |
dispersion degree D/v | 2.33 | 4.30 | 4.98 |
correlative coefficient r2 | 0.955 | 0.968 | 0.962 |
From table 1 we obtain the following conclusions:
The correlative coefficient are close to 1, this shows that the result of fitting parameter is reliable. Retardation factor of three soil are larger than 1, this shows that the adsorption property of F- is stronger in three soils. The higher the proportion of kaoline, the stronger adsorption capability of F-. Dispersion degree denotes dispersion intensity during solute transportation. According to fitting parameter in mid-coarse sand and kaoline mix of different proportion, we know that the higher the proportion of kaoline , the larger dispersion degree is, so the stronger adsorption capability of F- is.
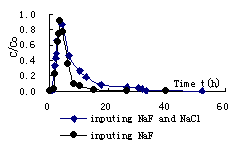
Fig.2 Breakthrough curve of F- in mid-coarse sand
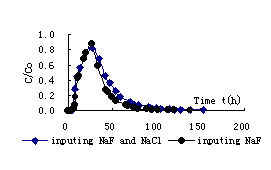
Fig.3Breakthrough curve of F- in mid-coarse sand and kaoline mix (1:10)
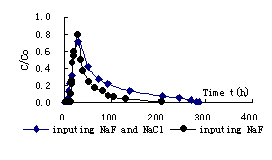
Fig.4 breakthrough curve of F- in mid-coarse sand and kaoline mix (1:8)
Solute transport rule in porous media can be influenced by many factors. There are mainly three transport mechanism: convection, diffuseness and hydrodynamic dispersion. Convection is result of mechanics; diffuseness is heat movement of particle; hydrodynamic dispersion is arose by the change of flow velocity in soil pore. Furthermore, solute transport can be influenced by other factors, such as soil physical property , chemical adsorption reaction and solution stability and so on. The breakthrough curve of F- in above three soils are respectively illustrated in fig.2, fig.3 and fig.4 during input NaF and mixed NaF and NaCl. According to the result of fig.2, it is found that initial breakthrough time is all shorter during input NaF and mixed NaF and NaCl due to flow velocity is faster in mid-coarse sand. The maximum concentration appears at time of 3.62h for input NaF and 4.27h for input mixed NaF and NaCl. Because of clay content is smaller and is weak in adsorption for mid-coarse sand ,so the peak value of relative concentration arrive at 0.905 for input NaF and 0.865 for input mixed NaF and NaCl. The breakthrough curve of F- in mixed mid-coarse sand and kaoline (1:10) is illustrated in fig.3 during input NaF and mixed NaF and NaCl . When mixed kaoline for mid-coarse sand ,the initial breakthrough time of F- increases. The maximum concentration appears at time of 26.48h for input NaF and 27.13h for input mixed NaF and NaCl. The peak value of relative concentration arrive at 0.877 for input NaF and 0.823 for input mixed NaF and NaCl. The breakthrough curve of F- in mixed mid-coarse sand and kaoline (1:8) is illustrated in fig.4 during input NaF and mixed NaF and NaCl .When mixed kaoline for mid-coarse sand ,the initial breakthrough time of F- increases. The maximum concentration appears at time of 27.98h for input NaF and 28.82h for input mixed NaF and NaCl. The peak value of relative concentration arrive at 0.794 for input NaF and 0.712 for input mixed NaF and NaCl.
The former research shows that the content of water-soluble fluorine in soil is closly related to fluorine content in groundwater and distributing of dental and skeletal fluorosis. The factors affecting water-soluble fluorine content have mainly PH-value ,organic matter ,clay content in soil and the proportion of calcium sodium ion in solution and so on. The chemical property of F- is greatly different from that of Cl- although both belong to same generic element. The chemical property of Cl- is not active , the retardation factor is usually smaller than 1 or equal to 1 due to repellency of soil electric charge during transport in earth column. But the chemical property of F- is active, so water-soluble fluorine in soil is easier to transport .Soil properties and other environment factors influence fluoride migration and its intensity. Li Chenghou’ study result shows that water-soluble fluorine has a negative correlation with the amount of <
In summary,Under the same experimental condition, coefficient of hydrodynamic dispersion and dispersion degree for fluorine in mid-coarse sand are smaller than that in mid-coarse sand and kaoline mix according to different proportion, this shows that the higher the content of clay particle possesses, the larger dispersion intensity is. This proves that soil which the content of clay particle is higher adsorp capability of F- increasly.
For mid-coarse sand and mid-coarse sand and kaoline mix according to different proportion, the difference of the breakthrough curve before maximum concentration is not obvious, only the time of appearingmaximum concentration and the value of maximum concentration differ slightly between input mixed NaF and NaCl solution and input NaF solution. This may be the result of competitive adsorption between F- and Cl-.During input water, sodium ion in solution accelerates migration and desorption of fluorine ion, so leaching velocity of F- during input mixed NaF and NaCl solution is faster than that during input NaF solution .
References
[1] Xie Zhengmiao,Wu Weihong. Translocation and transformation of fluorides in the environment and their biological effects[J]. Advances in Environmental Sciences, 1999,7(2):40-53.
[2] Wu Dunao,Yuan Fasong, Experimental Study on Fluoride Migration in Soil-Water System in the Region of BanShan of HangZhou[J].Environmental Pollution & Control,1990,12(2):5-9.
[3] Jiao You,Wei Kexun. Study on
[4] Zhang Naiming, Distribution of Fluorine and Its Affecting Factors in Soil in
[5] Yang Junyao, The Study on Dynamic Characteristics of Fluorine Migration in Unsaturated Soil[J].Journal of TaiYuan University of Technology,2000,31(2):107-109.
[6] Liu Zhaochang,Zhang Lansheng,Nie Yongfeng, Pollution and Control in Groundwater System[M].Peking,1991,225-232.
[7] Li Yunzhu,Li Baoguo.Solute transport in Soil[M]. 1998.113-130.
[8] Li Chenghou,Wan Hongyou . Preliminary Study on the Water-Soluble Fluorine Content in Soil and the Factors Affecting It[J].Journal of Mountain Agriculture and Biology, 2003, 22(2):99-104.
[9] Yang Junyao, Analysis of Influential Factor for Fluorine Migration in Water-Soil System[J]. Investigation & Surveying. 1998(3):42-44.
[10] Zhu Jicheng.Preliminary Study on Transport and Transformation of Fluorine Pollution in Groundwater, [J]. Environmental Chemistry, 1988,7(3):62-66.




