Achievements
REMOVAL OF HUMIC ACID FROM GROUNDWATER BY ELECTROCOAGULATION
Feng Qiyan, Li
Xiangdong, Cheng Yujie, Zhou Lai
China
University of Mining and Technology,
Abstract: Humic acid is popularly exiting in subsurface water and is harmful
to human health. In this study the effective performance of electrocoagulation
by sacrificial aluminium electrodes in the treatment of groundwater with humic
acid was investigated. Several working parameters, such as current density,
humic acid concentration and pH value were studied. The experiment results
showed that the smaller inter-electrode distance, the higher current density
enhanced removal rate of humic acid. Under acdic condition, when initial humic
acid concentration was set 20mg/L and current density was 47.6A/m2,
the effluent humic acid concentration was 0.43mg/L. The removal rate reached
99.8%. The results indicated that humic acid in groundwater can be removed
effectively by electrocoagulation. The removing mechanism
includeselectrocoagulation-adsorption, net rooling-sweeping and
electro-oxidation action.
Key words: Groundwater, Humic
Acid, Electrocoagulation, Aluminum Electrode, Removal rate; electrochemistry
1
Introduction
A major fraction of the natural organic matter (NOM) in surface or
ground waters is composed of humic acid (HA), and which is complex
macromolecular product of the chemical and biological degradation of plants and
animal residues. HA is a substance which is amorphous, brown or black,
hydrophilic, acidic and dispersive molecular weight. Humic acid adsorbed on the
surface of particles can alter their surface properties. Increase of humic acid
uptaking were assumed to be the neutralization of surface charges and the
compression of diffuse double layers. According to its properties the familiar
treatment on humic acid is adsorption, coagulation, micro-filtration and so on [1-3]. But the removal
efficiency for humic acid of these methods are not obvious, so it is important
to study new technique to remove humic acid for the safety of drinking water.
In the past decades electrocoagulation has been applied for the
treatment of many kinds of wastewater [4-7]. When aluminum is used as
electrode, the electrolytic dissolution of the aluminum anode produces Al3+,
which is transformed initially into Al(OH)3 and finally polymerized
Al→Al3++3e (1)
Al3++3H2O→Al(OH)3+3H+ (2)
nAl(OH)3→Aln(OH)3n (3)
Aln(OH)3n+mF-→AlnFm(OH)3n-m+mOH- (4)
Main reaction in cathodic compartment is as follows:
2H2O+2e→H2+2OH- (5)
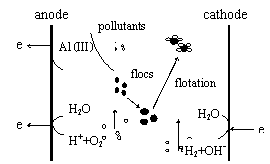
Fig. 1 Main stages involved in the electrocoagulation[10]
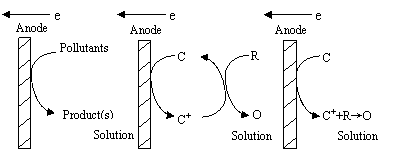
Fig. 2 Schematic representation of direct and indirect electro-oxidation process
The main stages involved in the electrochemically assisted
coagulation are shown in Figure 1. A generalized scheme for direct and indirect
electro-oxidation processes was demonstrated in Figure 2.
According to researches before humic acid in water are generally
removed by electrocoagulation-adsorption, net rooling-sweeping and
electro-oxidation action.
2
Materials and methods
Humic acid for experiment is produced by Tianjin Chemical Industry
Research Institute. The stock solution was prepared by dissolving the required
amounts of humic acid in deionized water. The pH value of the solution was
adjusted to 10 with 0.1mg/L sodium hydroxide. The concentration of humic acid
was analyzed by using Ultraviolet Spectrophotometer (UV-3100). The humic acid
concentration has a good relativity with absorbency at 254nm (y=42.0x-0.083,R2=1) [11]. Different concentration and pH value of humic acid
solution can be confected, and pH value of solution was tested by WTW526pH
meter.
The experimental setup is schematically shown in Figure 3. The
setup was made of plexiglass Aluminum plate was chosen as electrodes (it can be
embedded several electrode planks and the inter-electrode distance is changeable)
each with a dimension of
The water sample was injected from the top of the reactor
and flow out in a given
reaction time. The effluent solution filtrated by 0.45μm microfiltration
membrane was analyzed by ultraviolet spectrophotometer at a wave length of 254
nm. The removal rate of humic acid was calculated by the following formula:
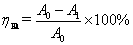 (6)
(6)
Where ηHA stand
for removal rate of HA, A0 is absorbency of solution before treatment, A1 is
absorbency of solution after treatment.
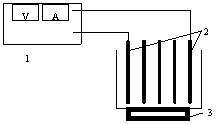
Figure 3 Schematic
diagram of experimental set-up
1. direct current power supply 2. electrodes
3. magnetic stirrer
3 Results and discussion
3.1 Effect of
inter-electrode distance
When current density was 20.5A/m2, the humic acid initial
concentration was 10mg/L the removal efficiency of humic acid under different
inter-electrode distance was shown in Figure 4.
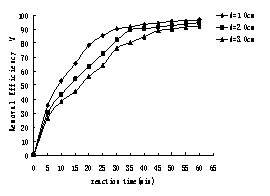
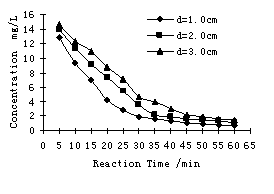
Fig. 4 Effect of inter-electrode distance on HA removal
The results indicated that the removal process was more efficient
with the inter-electrode distance being decreased. When the inter-electrode
distance was
3.2 Effect of
influent pH
The influent pH value is one of the important factors affecting
the performance of electrochemical process. The influence of pH on the
electrocoagulation was tested on the synthetic solution by simple addition of
acid (HCl) or alkaline solution (NaOH). In experiment pH value ranged from 3.0 to 9.5. When current density
was
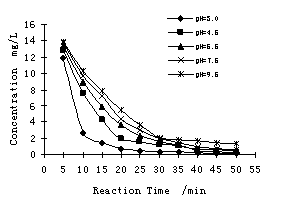

|
Reaction time/min
Fig. 5 Result of
humic acid removal under different pH value
The results indicate that the reaction was quick and the removal
efficiency was high when the initial pH was low, while the removal rate
decreased and removal efficiency reduced gradually with pH increased. This is
mainly because of the pH
influence on appearance of humic acid directly.
Aromatic ring is the basic unit of humic acid, and it is a
reticular macromolecule polymer which connected by hydrogen bonds between
functional retention. The most of active
functional retentions are carboxyl and phenolic hydroxyl group, and
dissociation of H+ relates to pH value of solution. When pH value is lower,
carboxyl and hydroxyl radical exist in -COOH and -OH form respectively, When pH
value is higher, they can exist in -COO- and -O- form. It is obvious that when pH value is higher, humic acid takes
more negative charge, and it needs more Al3+ to neutralize the negative charge.
So treatment effect and efficiency are all descended under higher pH value.
3.3 Effect of current
density
When the inter-electrode distance was 1.0cm, and humic acid
initial concentration was 20mg/L, the relation between removal efficiency and
current density presented in Figure 6. As expected, for a given time the removal
efficiency increased significantly with increasing of current density. When the
current density was 47.6A/m2 , about 94.6 % of HA was removed after 25min
reaction but it needed much more time under lower current density. This
indicated that at high current density more aluminum ion was produced by
electrolysis. And then aluminum hydroxide improved electrocoagulation.


Fig. 6 Effect of current density on humic acid removal
4
Conclusions
The experimental results showed that inter-electrode distance,
current density and influent pH are important variables that affect humic acid
removal efficiency. The inter-electrode was smaller and the current density was
higher, then the removal rate was higher. In experimental condition the removal
efficiency decreased with increasing of pH value. When current density was
47.6A/m2 the effluent humic acid concentration decreased from 20mg/L to
0.43mg/L, the removal rate reached 99.8%. The results indicate that humic acid
in groundwater can be removed effectively by electrocoagulation.
Acknowledgement
The authors would like to thank Prof. Zhao Xuan for useful discussions and advices and Li Jingbo for her experimental help.
References
[1] Aiguo Liu, Richard D.
Gonzalez. Adsorption desorption in a system consisting of humic acid heavy
metals and clay minerals[J]. Journal of Colloid and Interface Science, 1999,
218(1): 225–232.
[2] Christian Volk, Kimberly Bell, Eva Ibrahim, et al. Impact of
enhanced and optimized coagulation on removal of organic matter and its
biodegradable fraction in drinking water[ J]. Water Research, 2000,34(12):
3247-3257.
[3] Wei Yuan, Andrew L. Zydney. Humic acid fouling during
microfiltration [J]. Journal of Membrane Science, 1999,157 (1):1-12.
[4] Mollan M. Y. A., Morkovsky P., Gomes J. A. G., et al.
Fundamentals, present and future perspectives of electrocoagulation [J].
Journal of Hazardous Materials, 2004, B114(1-3): 199-210.
[5] Alinsafi A., Khemis M., Pons M. N., et al. Electro-coagulation of
reactive dyes and textile wastewater [J]. Chemical Engineering and Processing,
2005, 44(4):461-470.
[6] Yilmaz A. E., Boncukcuoglu
R., Muhtar Kocakerim M., et al. The investigation of parameters affecting boron
removal by electrocoagulation method [J]. Journal of Hazardous Materials, 2005,
B125 (1-3): 160-165.
[7] Gao P., Chen, X., Shen F., et al. Removal of chromium(VI) from
wastewater by combined electrocoagulation-electroflotation
without a filter [J]. Separation
and Purification Technology, 2005, 43 (2): 117-123.
[8] Hu C Y, Lo S L, Kuan W H, et al, Removal of fluoride from semiconductor
wastewater by electrocoagulation-flotation. Water
research, 2005, 39(5): 895-901.
[9] Buffle J, Parthasarathy N, Haerdi W. Importance of speciation methods in
analytical control of water treatment process with application to fluoride
removal from waste water. Water research, 1985, 19(1): 7-23.
[10] Canizares P., Carmona M., Lobato J., et al. Electrodissolution of
aluminum electrodes in electrocoagulation [J]. Industrial and Engineering
Chemistry Research, 2005, 44(12): 4178-4185.
[11] Lee M. C., Snosymk V. L., Crittenden J. C.. Activated carbon adsorption of homic
substance[J]. J A W W A, 1981, 73 (8) : 440-447.




