Achievements
TREATMENT OF TETRACHLOROETHENE WITH NANOSCALE NI/FE AND CU/FE BIMETALLIC PARTICLES
Huang Yuan-ying1, Liu Fei2, Shen Zhaoli2
1 National Research Center for GeoAnalysis,
2
Abstract:The research presented the effectiveness of nanoscale bimetallic particales( Ni/Fe and Cu/Fe) used to remove tetrachloroethene(PCE).Specific surface area of the laboratory synthesized nanoscale particles was approximately
Keywords: nanoscale bimetallic particles; reaction rate constant KSA; tetrachloroethene; dechlorination
1 Introduction
Because of many chlorinated solvents,such as tetrachloroethene(PCE),trichloroethene(TCE),dichloroethene(DCE) and vinyl chloride(VC),are known or potential threats to public health and the environment,so there is an urgent need to develop effective treatment methods. A survey of groundwater in a northern
In the last two decades,Fe0 permeable reactive barriers technology has received much interest as an alternative to pump and treat systems. As contaminated water passed through the permeable wall of iron to form primarily benign compounds such as hydrocaron and chloride ion. In general,reactions between chlorinated organic compounds(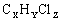 ) and iron in aqueous solutions could be expressed by the following reaction(Zhang,1998):
) and iron in aqueous solutions could be expressed by the following reaction(Zhang,1998):
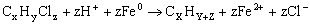 (1)
(1)
However, many challenges still exist for implementation of the zero-valent metal technology(Vidic et al,1996).Nanoscale metal particles, with diameter in the range 1-100nm, are characterized by high surface area to volume ratio, high level of stepped surface, and high surface energy. Nanoscale metal particles could be injected directly to contaminated soils,sediments, and aquifers for in situ remediation chlorinated hydrocanbons, instead of building permeable reactive barriers in the subsurface.The nanoscale particles can be anchored onto activated carbon and zerolite for ex situ treatment of contaminated waters and industrial effluents(Wang,1997). In the aqueous solution, the nanoscale iron particles could remain suspended under very gentle agitation.Within a bimetallic system, the dechlorination rates is the most rapid when Pd severs as catalyst.It is well known that its cost for application in engineering is expensive because Pd is a noble metal.We presented low-cost nickle and copper substitute for noble metal Pd. Using laboratory-synthesized nanoscale Ni/Fe or Cu/Fe remove PCE. To our knowledge, the report on dechlorination of chlorinated hydrocarbons by nanoscale Ni/Fe or Cu/Fe was not too much. It would have theory and actual signification if we work on this study.
2 Material and Methods
2.1 Synthesis of nanoscale iron particles
An appropriate amount of 1.0mol/L FeCl3·6H2O(v/v=30%ethanol) was placed in a conical flask, and purged with pure nitrogen for two hours. Adding 1.6mol/L NaBH4 solution into the conical flask with 1.0mol/L FeCl3·6H2O(1:1 volume ratio ).The solution was mixed vigorously on the magnetic agitator under 22±
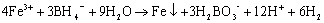 (2)
(2)
The nano-iron formed from the above reaction were rinsed with large volume of 0.5 mol/L HCl, de-oxygen water and ethanol for at least three times, respectively. Bimetals could be prepared by soaking freshly prepared nanoscale iron particles with ethanol solution containing 2% (wt.) NiCl2 or 4% (wt.)CuCl2. The mixture was stirred for 20 min and then filtered through a 0.2µm filter.This caused the reduction and subsequent deposition of Ni or Cu on the Fe surface:
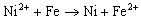 (3)
(3)
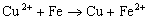 (4)
(4)
Ni/Fe and Cu/Fe particles were washed three times with ethanol and drying them at
2.2 Batch experiments
Batch experiments were conducted to test reactivity of the laboratory synthesized nanoscale particles for dechlorination of PCE.Take

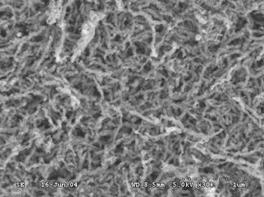
Fig.1 SEM image of nano-Ni/Fe(2%) Fig.2 SEM image of nano-Cu/Fe(4%)
Reactive materials | nanoscale iron particles(20~60nm) | micrometer particles(20~40mesh) |
BET surface area ( m2/g ) | 52.61 | 2.89 |
Note:BET data in Table 1were supplied by the lab of Analytical Chemistry in China University of Geosciences(Beijing).
2.3 Methods of analysis
Periodically,50uL of the aqueous solution was withdrawn by a 100-uL gas-tight syringe,and spiked quickly into a 10 mL vial charged with 0.45mL purified water.Concentrations of chlorinated ethenes were measured by a HP 6890GC equipped with a HP-5 capillary column (
GC HP-6890: inlet
Headspace sampler: vial
Method detection limits are about 0.05µg/L which is below the standards for the drinking water by the US EPA.Calibration curves for each model compound were made initially daily before analysis.
2.4 Chemicals
Five halogenated hydrocarbons dissolved in the methanol were purchased from the Institute of Reference Materials belonging to the Environmental Monitoring General Station of China, including 200µg/mL CF,50µg/mLCT,200µg/mL TCE, 100µg/mL PCE and 200µg/mL bromoform(BF).DCEs was from National Center for Reference Materials with a concentration of 0.93µg/mL.VC was from Chemistry Service, PA,USA. FeCl3·6H2O、NaBH4、NiCl2、CuCl2、HCl and anhydrous ethanol are analytical grade reagents.
3 Results and Discussion
3.1 Degradation of PCE with nanoscale Ni/Fe bimetallic particles
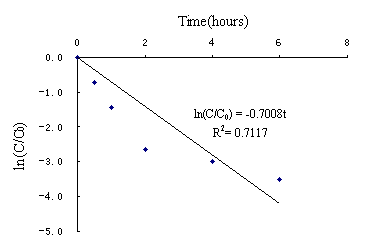
On the basis of forenamed method and processes, Fig. 3(a) showed the results of degradation of PCE by using nanoscale Ni/Fe bimetallic particles. No other product was detected with a reaction time(eg,TCE,DCEs and VC). ln(C/C0)-t were shown in Fig. 3 (b).Linear regression equation (1)was ln(C/C0)= -0.7008t, R2=0.7117(n=6).It indicated that the disappearance of PCE exhibited pseudo-first-order behavior.First-order rate constants Kobs=0.7008h-1,half-life time t1/2=ln2/ Kobs=0.99h.However,it took about 0.5h when reactive concentration C was up to half of initial concentration C0.According to reaction regression equation checkout, equation (1) was significative,but it distinctly deviated from the fact( Deng,1984). During the whole degradation process,it included two parts: firstly, in the first 2 hours, the concentration of PCE was reduced to 1.4mg/L from 21mg/L,that is, 93% of PCE was reduced using nanoscale Ni/Fe particles.As shown in Fig.3(c), linear regression equation(2)was C=21.07e-1..352t(0≤t≤2h),R2=0.9974(n=4).It indicated that the disappearance of PCE exhibited pseudo-first-order behavior. First-order rate constants Kobs=1.352h-1,half-life time t1/2=ln2/Kobs=0.51h, which was close to 0.5h.So equation (2) accorded with the experimental result.When iron specific surface area constant Pa was more than
3.2 Degradation of PCE with nanoscale Cu/Fe bimetallic particles
Fig.4(a) presented the results of degradation of PCE by using nanoscale Cu/Fe bimetallic particles.Unlike using nanoscale Ni/Fe where no chlorinated intermediates or final products, significant amounts of TCE were detected in the solution in runs.Within a 6-h, 90% of PCE was reduced using nanoscale Cu/Fe particles. ln(C/C0)-t were shown in Fig. 4 (b).Linear regression equation was (2)C=21.07e-0.377t, R2=0.9944(n=6).It indicated that the disappearance of PCE exhibited pseudo-first-order behavior.First-order rate constants Kobs=0.7008h-1,half-life time t1/2=ln2/Kobs=1.84h.Because there Pa was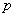 electron pair or
electron pair or 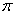 bond of chlorine atom in chlorinated hydrocarbons, and decreased the reactive energy.
bond of chlorine atom in chlorinated hydrocarbons, and decreased the reactive energy.
Fig.3 Degradation curve of PCE using nanoscale Ni/Fe
(a)
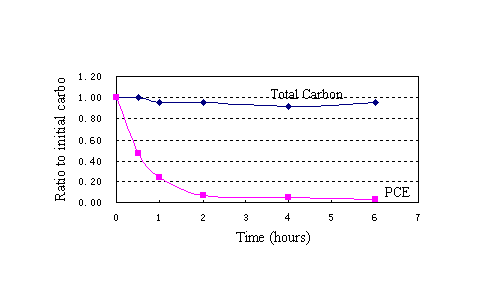
(b)
(c)
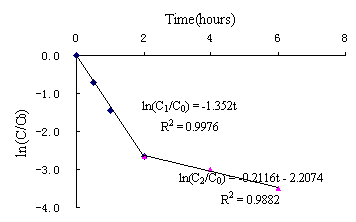
Fig.4 Degradation curve of PCE using nanoscale Cu/Fe
(a)
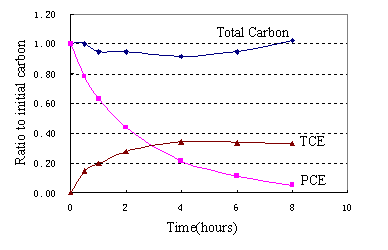
(b)

Table 2 Comparison on the reaction rate constant and half life time between different materials for dechlorination of PCE
Reactive materials | Kobs/h-1 | KSA/( mL·m-2·h-1) | t1/2/h | t50-N/h | Remark* |
Nanoscale Ni/Fe | 1.352 | 4.283 | 0.51 | 0.16 | 33.23 |
Nanoscale Cu/Fe | 0.377 | 1.194 | 1.84 | 0.58 | 9.26 |
micrometer grade Ni/Fe① | 0.1061 | 0.3697 | 6.53 | 1.87 | 2.87 |
micrometer grade Cu/Fe① | 0.0654 | 0.2279 | 10.60 | 3.04 | 1.77 |
micrometer grade Fe① | 0.0370 | 0.1289 | 18.73 | 5.38 | 1.00 |
Note:1)Mass ratio of nanoscale bimetals was 2%or 4% and metal to solution ratio was
2)①experimental data see also Table
3)* value listed were KSA ratio of using different materials to millimeter grade Fe under similar conditions.
4 Conclusions
In summary, nanoscale Ni/Fe and Cu/Fe particles had indicated distinct dechlorination for PCE. The degradation process appeared to be pseudo-first-order. Many advantages of a nanoscale bimetallic particles for treatment of PCE include:1)high spectific surface area and high surface reactivity, the nanoscale iron particles were observed to suspend in the aqueous solution, which favored to increase the dechlorination rate.Compared with micrometer grade iron,bimetallic Ni/Fe and Cu/Fe system, nanoscale bimetal for PCE exhibited higher activity, especially KSA of nanoscale Ni/Fe particles were improved 10~35 times.2) Due to the presence of catalyst Ni or Cu on the surface of iron,decreased reaction energy,increased the dechloriation rate,importantly reduced the production of byproducts. No other product was detected within a reaction time(eg,TCE,DCEs and VC) for the degradation of PCE with nanoscale Ni/Fe while significant amounts of TCE were detected in the solution with nanoscale Cu/Fe in runs.
Aknowledgement
This work was funded by the NSFC (40372109) and the project of Study on Remediation Technology of Soil Contaminated by Oil from the Beijing Education Commitee.The analytical data were supplied by the Lab of Water Resources and Environmental Engineering in China University of Geosciences (
References:
[1] Agrawal A, Trrtayek P G. 1996.Reduction of nitro aromatic compounds by zero-valent iron metal[J]. Environ.Sci.&Technol., 30(1):153-160.
[2] Deng B,Burris D R,Campbell T J. 1999.Reductive of vinyl chloride in metallic iron-water systems[J]. Environ.Sci.& Technol., 33(15): 2651-2656.
[3] Deng B. 1984.Application of Mathematical Statistics on analysis and test[M].Beijing:Chemical Industry Press. 149-185.
[4] Gillham R W, O’hannesin S F. 1994.Enhanced degradation of halogenated aliphatics by zero-valent iron[J].Ground water, 32:958-967.
[5] He X J,Liu F,HuangY Y,et al. 2003.Degradation of Volatile chlorinated aliphatics by zero valent iron[J].Environmental Science 24(1):139-142.
[6] Lien H L,Zhang W X. 2001.Nanoscale iron particles for complete reduction of chlorinated ethenes [J]. Colloids and Surfaces, 191:97-105.
[7] Liu F,Tang M G, He X J,et al. 2002. Laboratory study of chlorinated hydrocarbon in drinking water using Fe0[J],Earth Science-Journal of
[8] Liu F. 2002.Study on volatile chlorinated hydrocarbons in groundwater using the permeable reactive barrier of zero valence iron[D].a dissertation for doctor’s degree of China University of Geosciences(
[9] Liu F,Huang Y Y,Cui W H.2006.Impact factors on removal of perchloroethylene with nano-Ni/Fe methodology[J].Acta Geologica Sinica(English edition).80(2):262-266.
[10] Quan X, Liu H J,Yang F L,et al. 1998.Dechlorination of three polychlorinated hydrocarbons in water using bemetallic systems[J].China Environmental Science,18(4):333-336.
[11] Quan X,Yang F L, Xue D M,et al. 1997.Dechlorination of trichloroethylene in water by Pd/Fe0 catalytic reduction[J].Journal of
[12] R.D.Vidic,F.G.Pohland,1996.in:Technology Evaluation Report TE-96-01:Treatment Walls,Ground-water Remediation Technologies Analysis Center,
[13] Scherer M M,Balko B A,Gallagher D A.1998.Correlation analysis of rate constants for dechlorination by zero-valent iron[J]. Environ.Sci.& Technol.,32:3026.
[14] Mallat T, Bodnar Z, Petro J. 1991.Reduction by dissolving bimetals[J]. Tetrahedron,47(3):441-446.
[15] Yak H K,Wenclawiak B W,Cheng I F. 1999.Reductive dechlorination of polychlorinated biphenyls by zero-valent iron in subcritical water[J]. Environ.Sci.& Technol.,33(8):1307-1310.
[16] Wang C B, Zhang W X. 1997.Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs[J]. Environ.Sci.& Technol.,31(7):2154-2156.
[17] Zhang W X, Wang C B, Lien H L. 1998.Treatment of chlorinated organic contaminants with nanoscale bimetallic particles[J].Catalysis Today, 40:387-395.




